Understanding the underlying mechanisms related to charge transport (CT) across biomolecular junctions is imperative to design predictable biomolecular electronic devices. Although most studies ignore the interactions between a biomolecule and electrodes, a new study available in ACS Applied Materials and Interfaces focused on evaluating the net CT across the graphene-ferritin biomolecular tunnel junction.
Study: Temperature-Dependent Coherent Tunneling across Graphene–Ferritin Biomolecular Junctions. Image Credit: Gupta, K. N. et al., (2022) ACS Appl. Mater. Interfaces
A long distance, up to 10 nanometers (nm), across biomolecular tunnel junctions is one of the paradoxes of charge transport. This weak distance sensitivity indicates that the biomolecule-electrode interface could be one of the key variables associated with CT. There is a dearth of evidence that links biomolecule-electrode interfaces to CT efficiencies. Additionally, very few studies have elucidated how temperatures affect charge transport.
Development of Graphene-Based Biomolecular Tunnel Junction to Analyze CT
Graphene is an important bottom-electrode material that is used to immobilize biological molecules and form biomolecular junctions. Ferritins (AfFtn-AA) easily form a dense monolayer on graphene, which is used to investigate the mechanisms of CT.
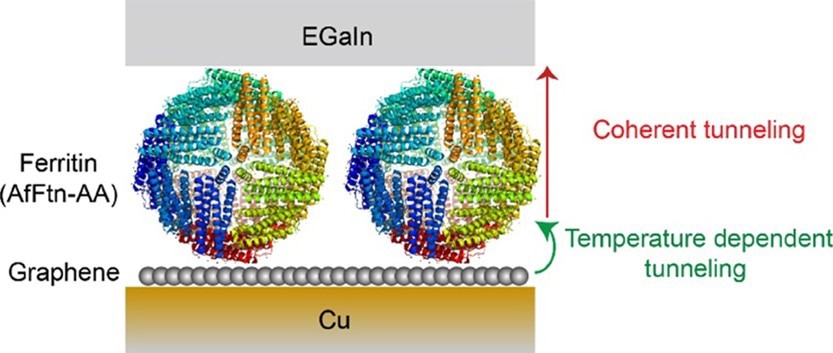
The copper/graphene//AfFtn-AA//GaOx/EGaIn tunnel junction was developed using the EGaIn technique. EGaIn is a liquid metal alloy composed of gallium (Ga) and Indium (In) in a 3:1 ratio. Additionally, AfFtn-AA, a globular protein, was isolated from Archaeoglobus fulgidus, a hyperthermophilic archaeon, and was used in this study.
One of the main advantages of using ferritin is that it can be loaded with iron oxide and has high thermal stability. To evaluate CT as a function of iron oxide nanoparticle loading, cone-shaped EGaIn tips were used as the top electrode. AfFtn-AA with no Fe-ion loading was used as a control to evaluate CT across biomolecular junctions.
A single bilayer of graphene, formed via the chemical vapor deposition (CVD) method, was used, with a copper foil to support the bilayer. The AfFtn-AA monolayer was formed on the Cu//graphene substrates, which contained iron oxide inside the cavity.
Graphene: A Potential Platform to Analyze CT Across Proteins in Tunnel Junction
The new study reports graphene as a potential platform for evaluating CT across proteins in biomolecular tunnel junctions. Importantly, it was observed that charging at the graphene and AfFtn-AA interface was dependent on the amount of iron oxide loaded.
Mechanistically, CT across biomolecular junctions has been categorized into three major groups: incoherent tunneling, resonant tunneling, and off-resonance tunneling. Biomolecules possess a low tunneling decay coefficient (β) over a distance greater than 4 nm; hence, long-range tunneling is impossible through coherent processes.
The interfaces in CT across biomolecular junctions contribute to major functions, including molecule-electrode coupling strength, energy alignment, and the number of available charge carriers. The interfacial charge that typically exists between biomolecules and electrodes is significantly more in the presence of highly charged proteins.
Although the interfacial dipole layer does not influence charge transport, as it extends insignificant depth, strong polarization reduces the coupling strength of the molecular wires and between doped silicon and bacteriorhodopsin. It is important to consider charge carriers during electronic applications. Graphene plays an important role in this context owing to its minimal number of carriers and its Dirac point.
The Behavior of CT Across Biomolecular Junctions
A tunneling behavior of biomolecules was observed when AfFtn-AA was directly adsorbed on graphene supported by copper. The present study reported two CT regimes, i.e., a high β for 600–3000 Fe loadings and low β for 3600–4800 Fe loadings. Importantly, a temperature-dependent CT activation was found in all Fe loadings and elevated energy barrier for higher Fe loading.
Cu//graphene//AfFtn-AA//GaOx/EGaIn junctions with cone-shaped tips of EGaIn were analyzed and the average current density was recorded. An increased average current density was observed that suggested enhanced net CT between ferritin and graphene.
Higher energy dispersion of graphene was associated with temperature; however, the CT transmission through AfFtn-AA was found to be temperature-independent. These findings imply that temperature-dependent conditions were predominantly caused due to interfaces. This observation could be true for other biomolecular tunnel junctions as well.
The model proposed by the current study, based on the evaluation of CT across Cu//graphene//AfFtn-AA//GaOx/EGaIn junctions, exhibited that temperature dependency linked to CT originated from the graphene–AfFtn-AA interface and not from incoherent tunneling across AfFtn-AA. The increase in Fe loading led to an enhancement in the tunneling distance.
The current study underscored the importance of the electrode-protein interface and pointed out that non-metallic electrodes might confer different features because they lower the electrode hybridization and alter the molecular state.
Reference
Gupta, K. N. et al. (2022) Temperature-Dependent Coherent Tunneling across Graphene–Ferritin Biomolecular Junctions. ACS Applied Materials and Interfaces. https://pubs.acs.org/doi/10.1021/acsami.2c11263
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.