Various lead halide perovskite devices have shown sensitivity to moisture content, thus impacting their physical properties. Humidity has been reported to degrade lead-halide perovskites and their devices.
Study: Nanomechanical signatures of degradation-free influence of water on halide perovskite mechanics. Image Credit: Niethammer Zoltan/Shutterstock.com
An article published in Communications Materials presented the effect of humidity on hardness and elastic modulus (E) for two series of lead halide perovskite single crystals. The results indicated the influence of hydrogen (H)-bonding, bond length, and polarization of the ions in lead halide perovskite single crystals.
The results revealed a curious inverse relation between hardness and modulus, which was strengthened with increased humidity. The present work's findings provided the material's distinct structure and properties at the atomic scale. The conclusion of this work was based on the evaluation of outcomes of various nano-indentation techniques that differentiated between surface and bulk E and explored different manifestations of hardness.
Hybrid Halide Perovskites
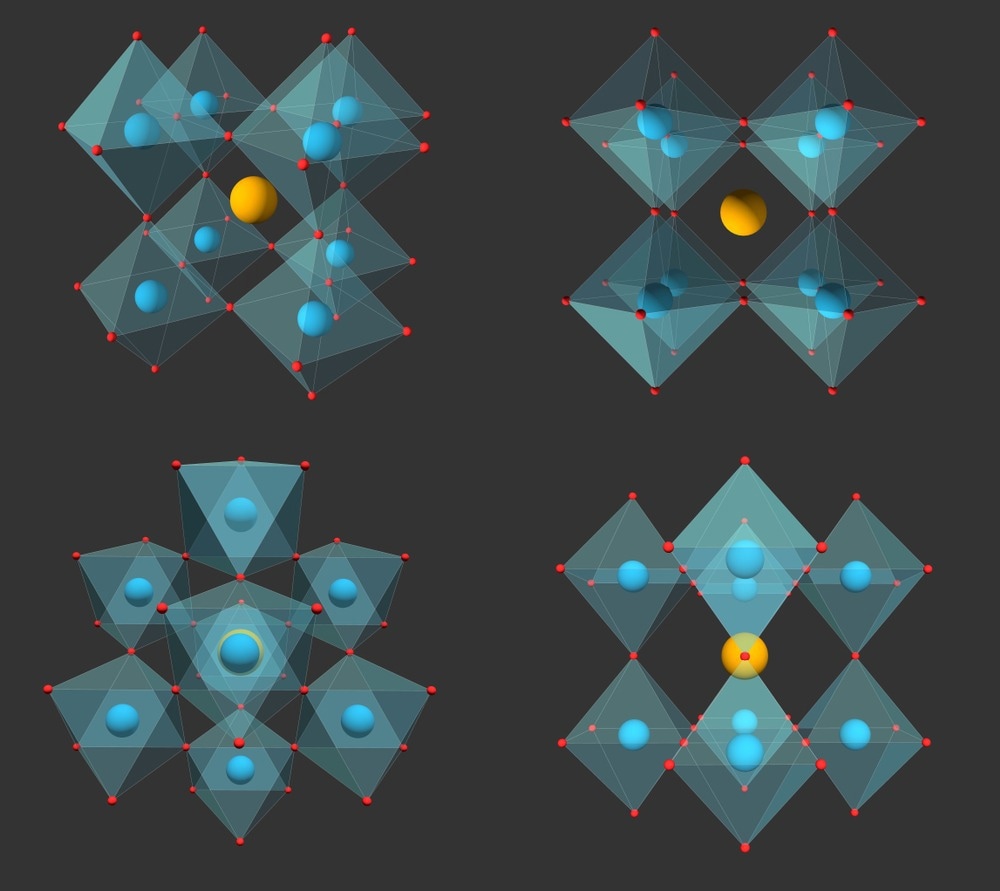
Halide perovskites, represented by ABX3 stoichiometry (where A, B, and X denote monovalent cations, divalent cations, and halide mono-anions, respectively), have outstanding optoelectronic properties.
The semiconductor industry relies on halide perovskites for various technologies, including wearable electronics, radiation detectors, photovoltaic solar cells, and light-emitting diodes. The mechanical properties of halide perovskites influence the non-radiative recombination and ion migration dynamics at strain-induced defects, necessitating an understanding of the halide perovskite mechanics.
Previous reports have hypothesized that material deterioration and mechanical changes vary with the exposure time to humidity. Although brief contact with humidity induces the production of reversible monohydrate in methylammonium lead iodide (MAPbI3), prolonged exposure promotes the formation of dihydrate, causing its irreversible breakdown into lead iodide (PbI2).
The deterioration of materials is an intricate process that may occur over a long period, even in inert atmospheres. Although the mechanism by which water (H2O) penetrates the crystal is still unknown, previous reports have shown that methylammonium ions (MA+) dissolve in excess water.
Nevertheless, an atomistic computational study based on H2O -MAPbI3 interactions did not indicate any chemical interactions between MA+ and H2O, suggesting that excess H2O is required to distort the lattice and induce degradation.
A polycrystalline thin-film-based experimental study validated the absence of water-MA+ interactions under ambient conditions (50% relative humidity). However, monohydrate formation was observed after several minutes at high relative humidity (>95%). Moreover, the sample porosity also influenced the water absorption, indicating that more porous crystals had low stability.
Effect of Humidity on Nanomechanical Properties
Exposing methylammonium lead halides (MAPbCl3, MAPbI3, MAPbBr3) and formamidinium lead bromide (FAPbBr3) to medium relative humidity did not degrade the materials, but their E value increased by 10%, compared to dry conditions.
The unusual increase in E value in the materials exposed to relative humidity was attributed to the underlying structural characteristics that differ for the bulk and the surface of the exposed material. The effect of humidity on E at the material's surface was due to the interaction of anions and cations with H2O, expressed as polarizability/polarity. Additionally, the effect of humidity on E in the bulk of the material was based on H-bonding and bond length.
A substantial increase in E was observed at the surface of MAPbI3 and in the bulk of FAPbBr3. The increased E in the latter was attributed to the diffusion of H2O when exposed to humidity. Moreover, the mechanical properties of cesium lead bromide (CsPbBr3) were independent of humidity, suggesting the critical role of H-bonding.
On the other hand, the effect of humidity on hardness contrasted with that of E, wherein the value decreased by 30% at higher humidity. This decrease in hardness was due to the accumulation of H2O within the perovskite framework and the formation of H-bonds with suitable lattice atoms when exposed to high humidity.
Conclusion
In summary, although exposing MAPbX3 and FAPbBr3 perovskites to a relative humidity of 55-60% did not degrade the lead halide perovskites, their elastic moduli were increased by 10% compared to the dry condition, and the effect was reversible.
The primary response observed in lead halide perovskites upon exposure to humidity was a decrease in the E, attributed to the inherent structural properties, which were distinct in the bulk and surface.
Furthermore, the modulus was related to fundamental material properties (hardness and bond stiffness). The hardness-humidity response was ascribed to H2O accumulation within the free space of the lead halide perovskite framework and H-bond formation between the lattice atoms. Thus, the present work evaluated and differentiated the surface and bulk E and analyzed various manifestations of hardness.
Reference
Buchine, I. et al. (2022). Nanomechanical signatures of degradation-free influence of water on halide perovskite mechanics. Communications Materials. https://doi.org/10.1038/s43246-022-00287-7
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.