The emergence and outbreak of the novel coronavirus, namely, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), resulted in a pandemic known as the coronavirus disease 2019 (COVID-19) pandemic. This pandemic has already claimed more than 6.7 million lives worldwide.
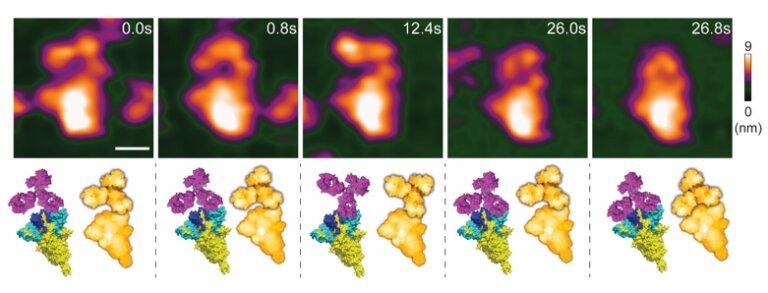
High-speed atomic force microscopy visualization of a spike-neutralizing antibody reacting with a spike protein. Credit: Lim et al
Several preventive and treatment measures have been designed to effectively manage COVID-19, including the recent development of anti-spike neutralizing antibodies (S NAbs). However, scientists have faced difficulties in the nanoscopic characterization of the vibrant interaction between spike proteins and S NAbs. A recent Nano Letters study characterized the molecular interaction between S NAbs and S proteins of SARS-CoV-2 using high-speed atomic force microscopy (HS-AFM).
The Role of S Protein in COVID-19
The spike (S) protein of the SARS-CoV-2 establishes the viral infection by binding with the angiotensin-converting enzyme-2 (ACE2) of the host and promoting the fusion of the membranes. It also triggers the host immune response to synthesize anti-spike neutralizing antibodies (S NAbs). S NAbs specific to NTD or RBD can be detected using high-throughput screening of antigen-specific B cells isolated from patients who recovered from SARS-CoV-2 infection.
In most SARS-CoV-2 variants, changes within the S proteins, particularly in the N-terminal domain (NTD) and receptor-binding domain (RBD), have been observed. For instance, the Delta variant contains L452R, K417N, and T478 K in the RBD, while the NTD contains G142D, T19R, and R158G, in the original SARS-CoV-2 strain. These changes in the S protein made the Delta variant more infectious, virulent, and able to escape immune responses.
Importance of Determining the Role of S NAbs in Preventing COVID-19
Research has shown that S NAbs can provide passive immunity for the treatment and prevention of COVID-19. They block the entry of SARS-CoV-2 by inhibiting interactions between the S protein and ACE2 of the host. Certain S NAbs are associated with antibody-dependent enhancement (ADE) by enabling the shedding of the S1 subunit after antibodies bind to RBDs. Subsequently, this shedding exposes the S2 subunit for proteolytic cleavage, promoting membrane fusion.
Scientists highlighted the need for real-time assessment of the dynamic nature of S NAb−S protein interactions at the nanoscopic range to determine the ADE risk. This cannot be achieved using cryogenic Electron Microscopy (cryoEM), as cryoEM imaging fails to determine the interactions between Fab fragments and S proteins. It also misses the significance of the Fc region and antibody bivalency. Nevertheless, the Fab fragment and the whole antibody (IgG1) exhibit varied outcomes in S protein-neutralization and elevating antibodies.
It is also essential to understand the nanoscopic occurrences of the dynamic interaction between the SARS-CoV-2 virus and S Nab to demonstrate the accessibility to S protein on the virus surface by S NAb.
HS-AFM- a Nanoscopic Assessment Platform of Anti-SARS-CoV-2 Spike Neutralizing Antibody
HS-AFM is an important nanoimaging tool with significant spatiotemporal resolution. The current study demonstrated that HS-AFM could be used for real-time imaging of the organelles, such as nuclear pore complex, conformational changes of viral fusion proteins, and histone H2A−DNA interactions, at a nanoscale range.
A nanoscopic assessment platform was developed, based on HS-AFM, to evaluate the binding property of an S NAb (MM43) and ADE risk. This nanoscopic assessment platform was based on the interactions between the S protein and MM43. Real-time imaging of the interactions between MM43 and S protein expressing sEV (S sEV) was conducted to mimic SARS-CoV-2 neutralization. HS-AFM real-time imaging demonstrated that MM43 moved and contacted SARS-CoV-2 wild-type (WT)- S sEVs and Delta-S sEVs. Furthermore, the MM43−S sEV interaction did not alter sEV topology and spatial parameters.
Notably, most S NAbs-based therapeutics developed for COVID-19 treatment belong to subclass IgG1. Hence, based on this information, the nanoimaging of an S NAb (MM43) was first performed using HAS-FM to elucidate the native IgG1 conformation and its conformational dynamics.
A mouse IgG1 crystal structure was simulated using the bioAFMviewer software to its HS-AFM images before initiating the scanning process. To identify a suitable substrate for MM43 adsorption, the net charge of IgG1 at neutral pH was analyzed using the Protein Tool on the Prot Pi Web site. PDB 2PQR19 and APBS20 analysis were used to generate a surface electrostatic map of IgG1. Bare mica was found to be the most suitable substrate for MM43 adsorption with maximum mobility. Nevertheless, during HS-AFM scanning, nickel-coated mica was found to perform better than bare mica for Y conformation imaging of MM43.
The binding pattern of MM43 to the recombinant S protein of the ancestral strain was studied under a physiological buffer. Here, one Fab region was firmly bound with the S protein, while the free Fab and Fc regions were dynamically moving during HS-AFM imaging. HS-AFM imaging revealed that the MM43 and S protein interaction did not trigger RBD opening, indicating that MM43 could have an insignificant risk of ADE.
Taken together, the current study developed a nanoscopic assessment technique based on HS-AFM imaging, which enabled the detection of the binding pattern of S NAb and the active interaction between S NAb and S sEVs. These findings will help identify suitable S NAbs for COVID-19 treatment.
Reference
Lim, K. et al. (2023) Nanoscopic Assessment of Anti-SARS-CoV-2 Spike Neutralizing Antibody Using High-Speed AFM. Nano Letters. https://doi.org/10.1021/acs.nanolett.2c04270
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.