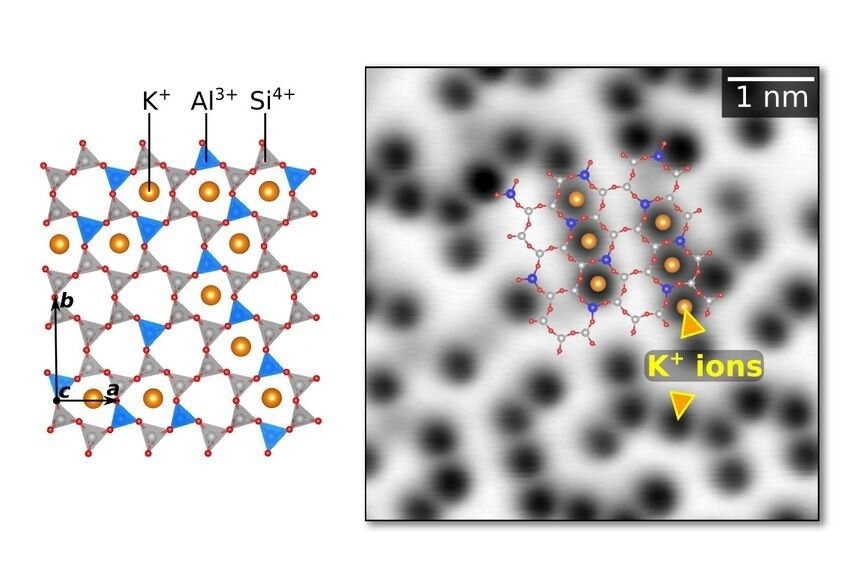
Muscovite mica is a common layered phyllosilicate mineral with perfect cleavage planes. These atomically flat surfaces are ideal for studying minerals and clays using scanning probe techniques with atomic resolution. However, there are still unresolved questions about ordering K+ ions on the cleaved surface.
Atomic structure of mica and a picture taken by an atomic force microscope. Image Credit: Vienna University of Technology
A recent study published in Nature Communications focuses on this problem by studying the K+ distribution on cleft mica surfaces using low-temperature and non-contact atomic force microscopy (AFM) in ultra-high vacuum (UHV).
Muscovite Mica: Overview and Significance
Muscovite mica is a common mineral widely used in surface and interface science research. This material is composed of layers of aluminosilicate and potassium ions and has perfect cleavage planes that allow for the creation of atomically flat surfaces. These surfaces are ideal for scanning probe techniques, which can provide atomic-resolution images of the mineral.
Over the past few decades, muscovite mica has been the attention of numerous studies across various fields, including environmental science, nanotribology, and developing next-generation electronics based on two-dimensional materials.
Mica's unique characteristics, including its atomically smooth surfaces and extensive understanding of its bulk qualities, have made it a popular model substrate in a variety of scientific situations.
Examples of research conducted on mica include studies on the adsorption and kinetics of biomolecules, as well as studies on water. These studies attempt to understand the atomic-scale principles behind the widespread water-mineral interplay on planet Earth.
Moreover, the flatness and simplicity of mica production have made it the preferred test system for novel experimental methods with real-space, molecular precision in atmospheric or liquid settings that go beyond the classic atomic force microscope (AFM).
Challenges and Limitations of Muscovite Mica
Despite its popularity and importance in surface and interface science, muscovite mica still poses many unresolved questions. Surface K+ ions have been a prominent focus because they may be readily transferred in solution and provide an intriguing playground for studying ion hydrolysis and ice formation on mineral surfaces.
Because of the lack of UHV direct imaging, the inherent K+ concentration at the surface remains unaltered by contact with the environment. The aluminum (Al) arrangement in the underlying tetrahedral sheets is another source of ambiguity. Since aluminum (Al) and silicon (Si) have comparable scattering coefficients in X-Ray diffraction, determining the empirical Al distribution is challenging.
The existence of Al short-range ordering has been postulated by early nuclear magnetic resonance (NMR) and Monte Carlo (MC) calculations. Through electrostatic contact, such ordering might influence the distribution of surface K+ ions. However, owing to the difficulties of analyzing intrinsic surface K+ distributions, proving this idea in atmospheric conditions remains a major challenge.
Highlights of the Current Study
In this study, the researchers used a methodology that included photographing the mica surfaces under ultra-high vacuum (UHV) conditions. This method was used to evaluate the inherent ordering of K+ ions and perhaps tie it to the subsurface Al ion distribution.
However, traditional UHV cleaving methods have often introduced strong electrostatic fields that have made advanced imaging techniques, such as atomic force microscopy (AFM), challenging. To overcome this obstacle, the authors used non-contact AFM imaging on UHV-cleaved, clean mica samples.
The results obtained from these imaging techniques were then analyzed using a combination of density functional theory (DFT) calculations and Monte Carlo (MC) simulations. The DFT calculations were used to understand the distribution of the K+ ions. In contrast, the MC simulations were used to gain more information on the Al3+ order based on the measured surface K+ order.
"We were able to see how the potassium ions are distributed on the surface," says Giada Franceschi, the first author of the current paper, who works in Prof. Ulrike Diebold's team. "We were also able to gain insights into the positions of the aluminum ions under the surface layer—this is a particularly difficult task experimentally."
Important Developments and Future Perspectives
This research provides new insights into the surface structure of mica, a widely studied mineral in surface and interfacial science. By using ultra-high vacuum (UHV) and advanced imaging techniques, the study was able to reveal the intrinsic short-range ordering of the surface K+ ions. These ions are found to arrange in short, alternating rows, contradicting previous assumptions of a random arrangement.
The results show that the K+ ordering is not only due to electrostatic repulsion between the ions but is also heavily influenced by the interaction with Al3+ ions in the subsurface aluminosilicate layer.
The atomic-scale insights provided by this study on UHV-cleaved mica broaden the current understanding of mineral surfaces and offer valuable input to disentangle the many factors at play in more complex ambient or liquid environments. This could lead to discoveries and applications in various fields, such as material science, chemistry, and geology.
Reference
Franceschi, G. et al. (2023). Resolving the intrinsic short-range ordering of K+ ions on cleaved muscovite mica. Nature Communications. Available at: https://doi.org/10.1038/s41467-023-35872-
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.