Recently, researchers at Yale University and the University of Connecticut collaborated to develop a nanoparticle-based treatment to fight glioblastoma (GBM), one of the most harmful malignancies with a high recurrence rate and poor clinical outcome. This newly developed technique targets multiple factors associated with GBM progression and invasiveness. The findings were published in Science Advances.
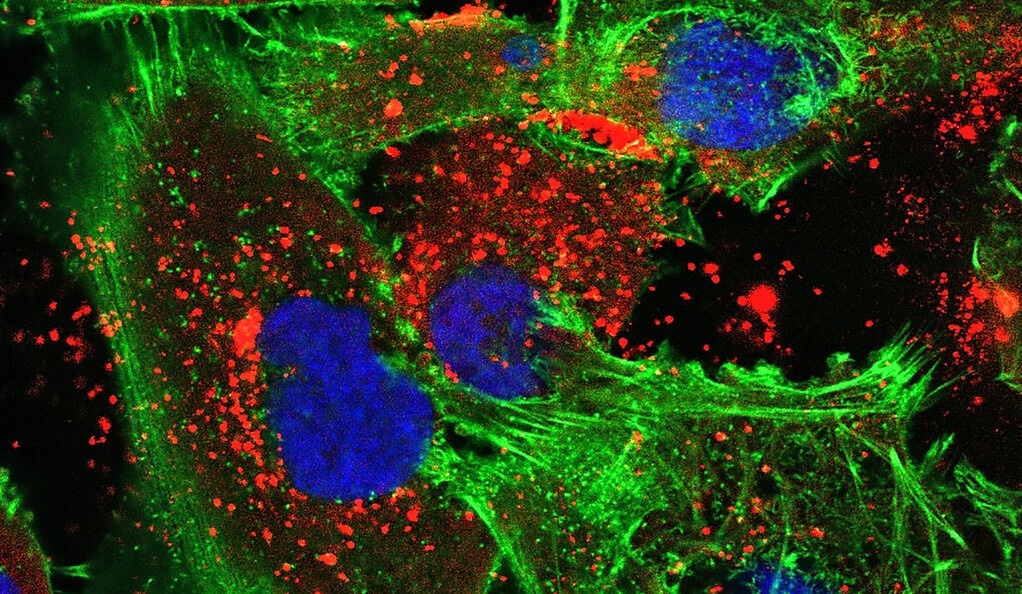
A new treatment developed by Yale researchers uses bioadhesive nanoparticles that adhere to the site of the tumor and then slowly release the synthesized peptide nucleic acids that they’re carrying. In this image, the nanoparticles (red) are visible within human glioma tumor cells (green with blue nuclei).
GBM: Cause and Conventional Treatment
Around 14.5% of nervous system tumors have been linked to GBM, with a survival rate of approximately 15 months. The incidence rate of GBM in the US is 4.32 per 100,000 persons a year, with a poor survival rate.
Conventional treatment of GBM includes surgery, followed by radio-and-chemo-therapy. Notably, temozolomide (TMZ), a chemotherapy treatment in combination with radiotherapy has improved survival rate by two years.
MicroRNAs (miRNAs) are approximately 25 nucleotides long non-coding RNAs involved with genetic expressions at the post-transcriptional level. Several studies have indicated that miRNA dysregulation, at an up-regulation (oncomiRNAs) or down-regulation, is a potential driver of malignancies.
Unusual miRNA expression levels were observed in patients with GBM, which resulted in poor prognosis and survival rates. For instance, miR-10b and miR-21 were identified to be the significantly up-regulated oncomiRs, manifesting GBM.
Mechanistically, miR-10b increases GBM growth by negatively regulating transcription factor AP-2γ (TFAP2C), cyclin-dependent kinase inhibitor I (p21) expression, BCL2 interacting mediator of death (Bim), and tumor suppressor cyclin-dependent kinase 2A inhibitor (CDKN2A/16). Similarly, GBM invasiveness is increased by up-regulated miR-21 levels.
Mechanistically, an up-regulated miR-21 inhibits matrix metalloproteinase (MMP) and stimulates cell proliferation via negative regulation of phosphatase and tensin homolog (PTEN) and insulin-like growth factor–binding protein-3 (IGFBP3). It also induces tumor stemness through SRY-box transcription factor-2 (SOX-2).
In vivo experiments revealed that miR-10b inhibition reduces intracranial GBM tumor growth, which ultimately prompted the development of antisense oligonucleotide (RGLS5799, Regulus Therapeutics) targeting miR-10b.
Alternatively, knocking down miR-21 decreases GBM advancement and invasion. This treatment also reduces GBM cell’s chemoresistance to TMZ and taxol. The available GBM therapeutics mainly target a single oncomiR, which has shown reduced efficacy.
A New Nano-based GBM Treatment
As stated above, scientists from Yale and the University of Connecticut have designed a nanoparticle-based treatment for GBM. This therapy targets both miRNAs, i.e., miR-10b and miR-21 simultaneously, to increase the chemosensitization of GBM toward TMZ.
In this study, bioadhesive nanoparticles were used, which contained newly synthesized peptide nucleic acids (PNAs). PNAs were able to actively regulate gene expression, particularly oncomiRs. They are synthetic nucleic acid analogs, in which the phosphodiester backbone is replaced with neutral N-(2-aminoethyl) glycine units. The newly developed bioadhesive nanoparticles adhere to the tumor site, slowly release the PNAs that target oncomiRNAs, and inhibit tumor-promoting activity.
Typically, PNAs bind to targeted miRNAs via a complementary DNA base pairing system, and this structure is enzymatically stable. However, compared to classical PNAs, serine-gamma PNAs (γPNAs), with specific modification at the γ position, exhibit superior binding affinity, physicochemical features, and specificity. Previous studies have also indicated that anti-seed sγPNAs are clinically more translatable with minimal toxicity.
The newly designed γPNAs are complementary to the seed region of oncomiR-21 and oncomiR-10b, to improve anti-miRNA activity. Besides its simplistic synthesis methodology, γPNAs are also ideal for conjugation with fluorophores or other probes, which are useful for imaging.
The convection-enhanced delivery (CED) system has been developed to directly introduce polymeric nanoparticles (NPs) loaded with active agents to brain tumors. In this study, the bioadhesive NPs (BNPs) comprised hyperbranched polyglycerol (PLA-HPG) and a copolymer of poly(lactic acid), ultimately forming PLA-HPG-CHO, which is highly beneficial to deliver PNA anti-miRs.
In this study, PLA-HPG-CHO BNPs were loaded with two sγPNAs, one bound to miR-10b and the other to miR-21. The Gel shift assays showed the binding of the synthesized sγPNAs (sγPNA-21 and sγPNA-10b) with the respective miR. This finding indicated that the newly designed sγPNAs were highly specific and had a strong affinity for target oncomiRs.
Compared to classical PNAs loaded in the PLA-HPG-CHO BNPs, sγPNAs loaded in PLA-HPG-CHO BNPs exhibited a greater miR inhibition. When sγPNAs loaded PLA-HPG-CHO BNPs were evaluated in a GBM challenged mice model, the treated mice lived longer compared to the control mice.
Notably, sγPNAs loaded PLA-HPG-CHO BNPs remained at the target site for about 40 days, which is extremely advantageous compared to conventional site-specific treatments that wane off fairly quickly.
In addition, as the current treatment knocks down both GBM targets simultaneously, it is more powerful than existing treatments. Mark Saltzman, a professor at the Yale Cancer Center, who was involved with this research, stated, “These results are the best I've ever seen in this sort of aggressive brain tumor.”
Reference
Wang, Y. et al. (2023) Anti-seed PNAs targeting multiple oncomiRs for brain tumor therapy. Science Advances. 9(6). DOI: 10.1126/sciadv.abq7459.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.