A new study published in the journal Nature Communications utilizes a rhodium nanocrystal surface with nm-sized nanofacets to model a compartmentalized reaction nanosystem. By examining this model, researchers hope to gain insight into the behavior of compartmentalized reactions in nanosystems.
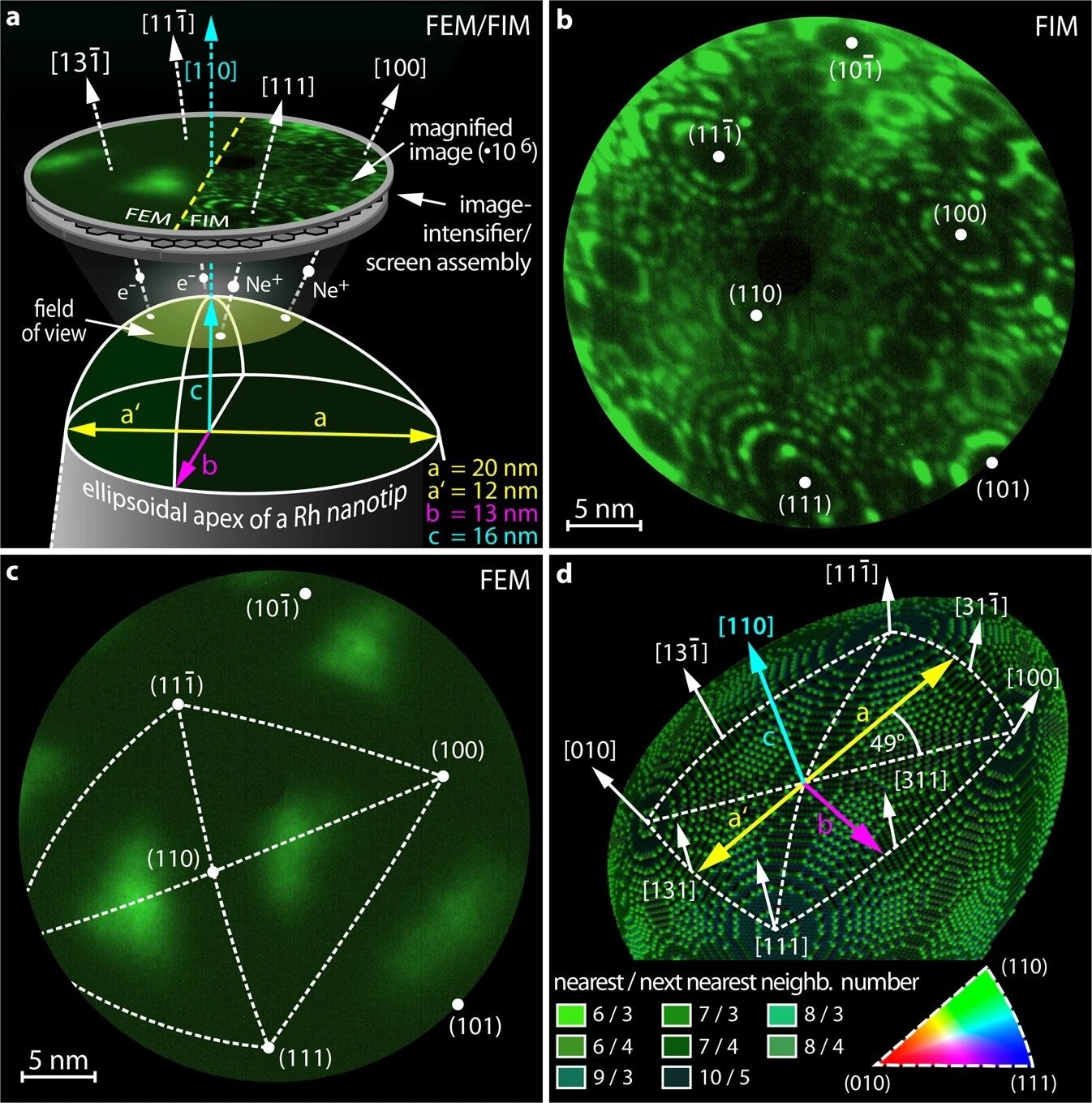
Apex of an ellipsoidal Rh nanocrystal. a Schematics of the experimental setup and the sample geometry: in FEM and FIM, field emitted electrons and ions, respectively, form a point projection image of the sample surface. The apex of the [110]-oriented Rh nanocrystal has axis lengths of a, a′, b and c. b FIM image of the Rh nanocrystal, obtained at T = 77 K using Ne+ ions. c the same field of view, but in the FEM mode, with the same crystallographic markings as in b. d Ball model of the nanocrystal apex with crystallographic net overlay, where each of the four triangles forming the net corresponds to the inverse pole figure (bottom right). To illustrate the local atomic corrugation, the individual atoms are color-coded according to their nearest and next-nearest neighbor numbers. Credit: Nature Communications (2023). DOI: 10.1038/s41467-023-36434-y
Chemical reactions can behave differently in adjacent compartments within compartmentalized systems. In nanosystems with compartments, these reaction behaviors can deviate from those observed on a larger scale. In situ studies of processes within such nanosystems are often challenging, making theoretical simulations the primary means of analysis.
Compartmentalization: The Fundamental Principle of Catalytic Reactions
Compartmentalization is a fundamental principle of life that plays a crucial role in enabling the chemical reactions that sustain living organisms. The design of cells as flexible compartment systems permits chemical reactions to occur in neighboring compartments in spatially diverse ways.
Remarkably, compartmentalization may be found in non-living systems as well as living organisms. For instance, oscillatory reactions in microemulsions, particle arrays, and gels are instances of compartmentalization in a non-living environment.
Another domain where compartmentalization may have a substantial influence is catalysis. Using a core-shell framework composed of two well-defined metal-oxide interfaces inside one particle or two-phase aquatic systems, mismatched catalytic reactions can occur simultaneously in close vicinity.
All of these phenomena occur in two-dimensional systems, which simplifies the understanding of the ongoing processes. However, while compartmentalization has been studied extensively, a complete atomistic comprehension of the underlying processes remains challenging. Corresponding micro-kinetic modeling provides a promising avenue for gaining insight into these complex systems.
Nanosized Compartments in Catalytic Systems: A Research Gap
Studying spatiotemporal phenomena on a microscopic level is essential for understanding the behavior of catalytic nanosystems. While researchers have previously investigated these phenomena on macroscopic single-crystal surfaces, it is important to explore compartmentalized systems to gain insight into how individual nanosized compartments operate.
However, current models that use polycrystalline metal foil cannot study individual compartments in detail. To address this issue, researchers have turned to the apex of a catalytically active metal tip as a model system.
This system allows in situ studies of individual nanosized compartments and provides a wide playground for exploring compartmentalization in catalytic nanosystems.
Although researchers have observed observable reaction instabilities such as kinetic transitions and self-sustaining oscillations, they have not yet observed a transition from synchronous behavior to chaos in a nanosized system. Thus, further research is required to explore the possibility of chaotic behavior in compartmentalized reaction systems.
Highlights of The Current Study
To investigate the compartmentalized reaction behavior, the researchers used two microscopy techniques: field ion microscopy (FIM) and in situ field emission microscopy (FEM). The team first employed FIM to determine the shape, size, and atomic structure of the individual nano-facets. The researchers then used FEM to observe the ongoing hydrogen oxidation at a resolution of about two nanometers.
The researchers varied the temperature and hydrogen pressure during the experiments and observed mono-frequential and multi-frequential self-sustained kinetic oscillations.
To complement the experimental studies, the researchers used microkinetic modeling to study hydrogen oxidation on rhodium (Rh) nano-facet compartments acting as coupled individual oscillators. The team also investigated chaotic behavior in a particular region of the pH2/T space caused by the breakdown of synchronization between compartments.
"The crystal consists of many different surface nano-facets, like a polished diamond, but much smaller, on the order of nanometers," explain Maximilian Raab and Johannes Zeininger, who performed the experiments. "On each of these facets, the chemical reaction oscillates, but the reactions on neighboring facets are coupled."
Key Developments and Future Avenues
The research findings revealed that the catalytic hydrogen oxidation in a compartmentalized reaction nanosystem, containing differently oriented rhodium nanofacets, occurs in parallel and exhibits distinct reaction modes, including a shift from self-sustaining oscillations to chaotic behavior.
The theoretical models agreed with the experimental observations, providing further evidence for the emergence of chaotic behavior in this nanosystem. It was found that the weakening of coupling in the system leads to chaos through spatial separation into several individually oscillating clusters.
The study also revealed the significance of diffusional coupling in nanosystems, which significantly complicates observing low-dimensional chaotic behavior.
The study is the first attempt to transfer the existing knowledge of chaos in complex dynamical systems to catalysis on a nanoscale. The findings provide insights into the complexity of heterogeneous catalytic reactions and the possibility of nanoscale confinement and chaotic behavior in small, compartmentalized chemical or biological systems.
Future studies can build upon these findings to explore the possibility of more realistic catalysts and complex reactions using appropriate observation techniques.
Reference
Raab, M. et al. (2023). Emergence of chaos in a compartmentalized catalytic reaction nanosystem. Nature Communications. Available at: https://doi.org/10.1038/s41467-023-36434-
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.