Researchers at Kanazawa University report in Analytical Chemistry how to produce nanopipettes that reliably provide nanoscale resolution scanning ion conductance microscopy images of living cells.
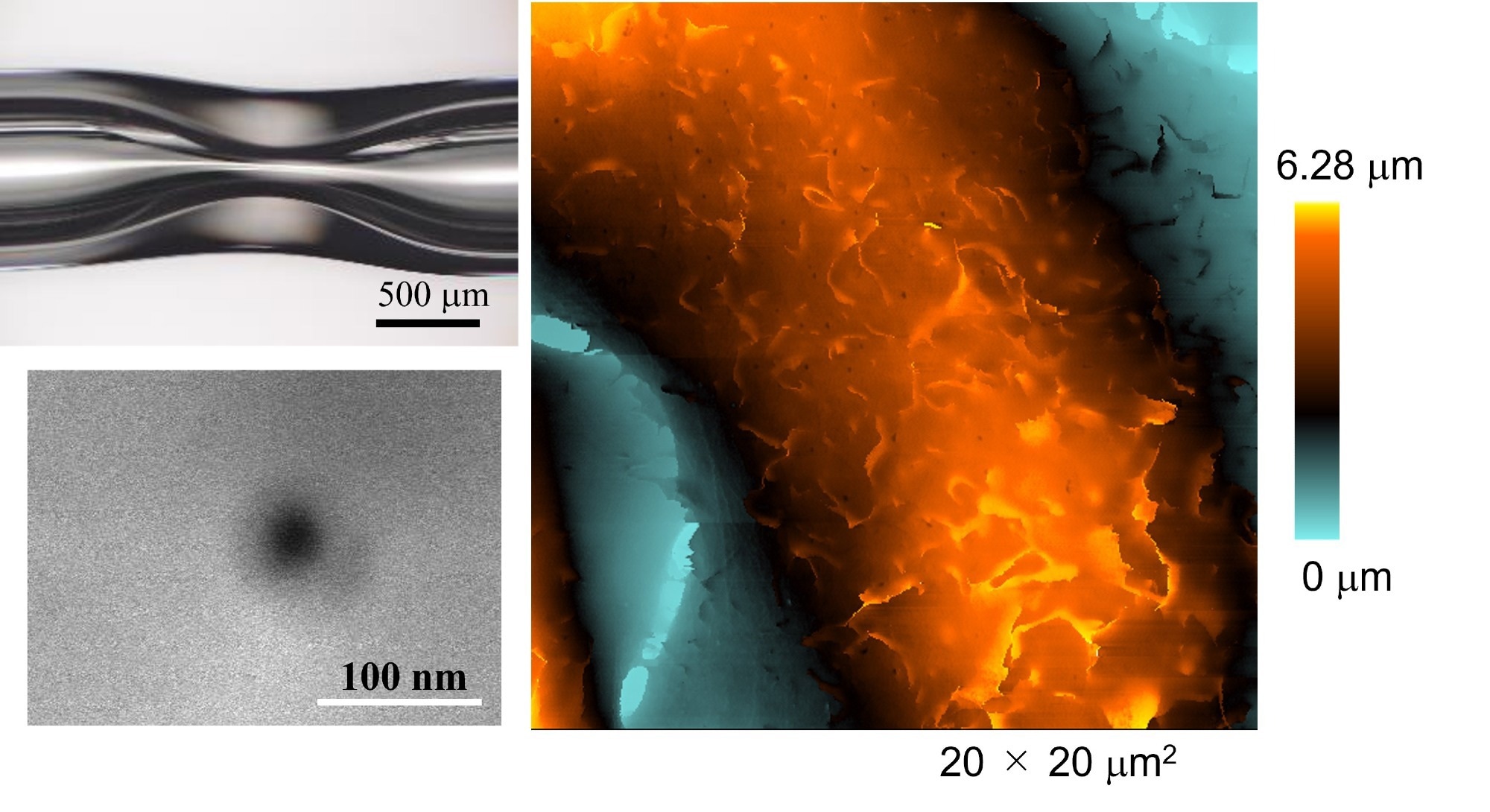 New method for fabricating nanopipette and ultra-high-resolution imaging of its shape. Upper left: Glass capillary after preheating to deform glass. Lower left: Electron micrograph of a nanopipette tip; Right: SICM image of the surface of a fixed HeLa cell. Image Credit: American Chemical Society
New method for fabricating nanopipette and ultra-high-resolution imaging of its shape. Upper left: Glass capillary after preheating to deform glass. Lower left: Electron micrograph of a nanopipette tip; Right: SICM image of the surface of a fixed HeLa cell. Image Credit: American Chemical Society
A nanoscale view of living cells can provide valuable insights into cell structure and function. Over the years, various microscopy techniques have been enrolled to obtain a window into biological specimens at the nanoscale but all with their limitations and challenges. Although scanning ion conductance microscopy (SICM) has demonstrated the capability to image living biological samples in solution with nanoscale resolution, it has been hampered by challenges in reliably producing nanopipettes with the optimum geometry for the job. Now researchers led by Yasufumi Takahashi at Kanazawa University's Nano LSI and Nagoya University have devised a protocol for reproducibly fabricating nanopipettes with the preferred geometry for high quality imaging. Scanning ion conductance microscopy (SICM) uses a nanopipette to control the nanopipette-samples distance using an ion current as feedback signal. The shape of the nanopipette significantly influences the performance of the device. For instance, a wide aperture limits the possible resolution, a long shunt can lead to rectification effects that warp the ion current measurements, and if the glass of the nanopipette is too thick it can deform the sample before the proximity of the aperture has reached the point needed for constant ion current topographical mapping. As a result, the ideal nanopipette has a short shunt, small aperture and thin glass walls.
The standard procedure for fabricating the nanopipette is to pull a capillary tube with a laser puller that heats the capillary tube it is manipulating. The capillary then narrows where it lengthens until it is finally drawn into two separate pieces. Although quartz can allow a little more control in the process of drawing the capillary tube into shape it is hydrophobic, which raises complications in actually filling the nanopipette with the aqueous solution needed for the ion current. In this reason, the researchers developed a protocol by which they could draw nanopipettes from borosilicate glass capillaries with the required control and reproducibility.
Takahashi and his collaborators noted that ideally the starting capillary should have thick walls and a narrow inner diameter, however it is not easy to obtain capillary tubes to these requirements from commercial suppliers. Instead, they preheat the capillary for 5 s without pulling it, which causes the glass walls to the thicken and reduces the inner diameter. They also optimized the parameters for pulling the tube, such as the velocity.
The researchers demonstrated the performance of the nanopipettes they produced by imaging a cell undergoing a type of endocytosis, where it engulfs and absorbs some external material. They were able to image the microvilli – cellular membrane protrusion - on the cell surface, the endocytic pits that form and the formation of a cap closing up the pit. Previously attempts to image the cap formation have been inhibited by limitations in the spatial resolution.
The researchers were even able to resolve extracellular vesicles as small as 189 nm released in the process. As they point out in their report there is increasing evidence that these extracellular vesicles play an important role in communication between cells and homeostasis, with diagnostic and therapeutic applications focused on the smaller extracellular vesicles between 40 nm and 150 nm in particular. The researchers conclude in their report, "We envision this protocol will help to reproducibly fabricate borosilicate nanopipettes for high-resolution topographical mapping using SICM."