In a groundbreaking development in cancer imaging, researchers from Johns Hopkins University have unveiled a pioneering approach to detecting and visualizing tumors with unprecedented precision. The newly introduced molecular nanoprobes, aptly named nanoSABERs, are designed to interact with specific enzymes found within cancer cells, shedding light on the intricacies of tumor behavior, which could offer earlier and more effective treatment strategies.
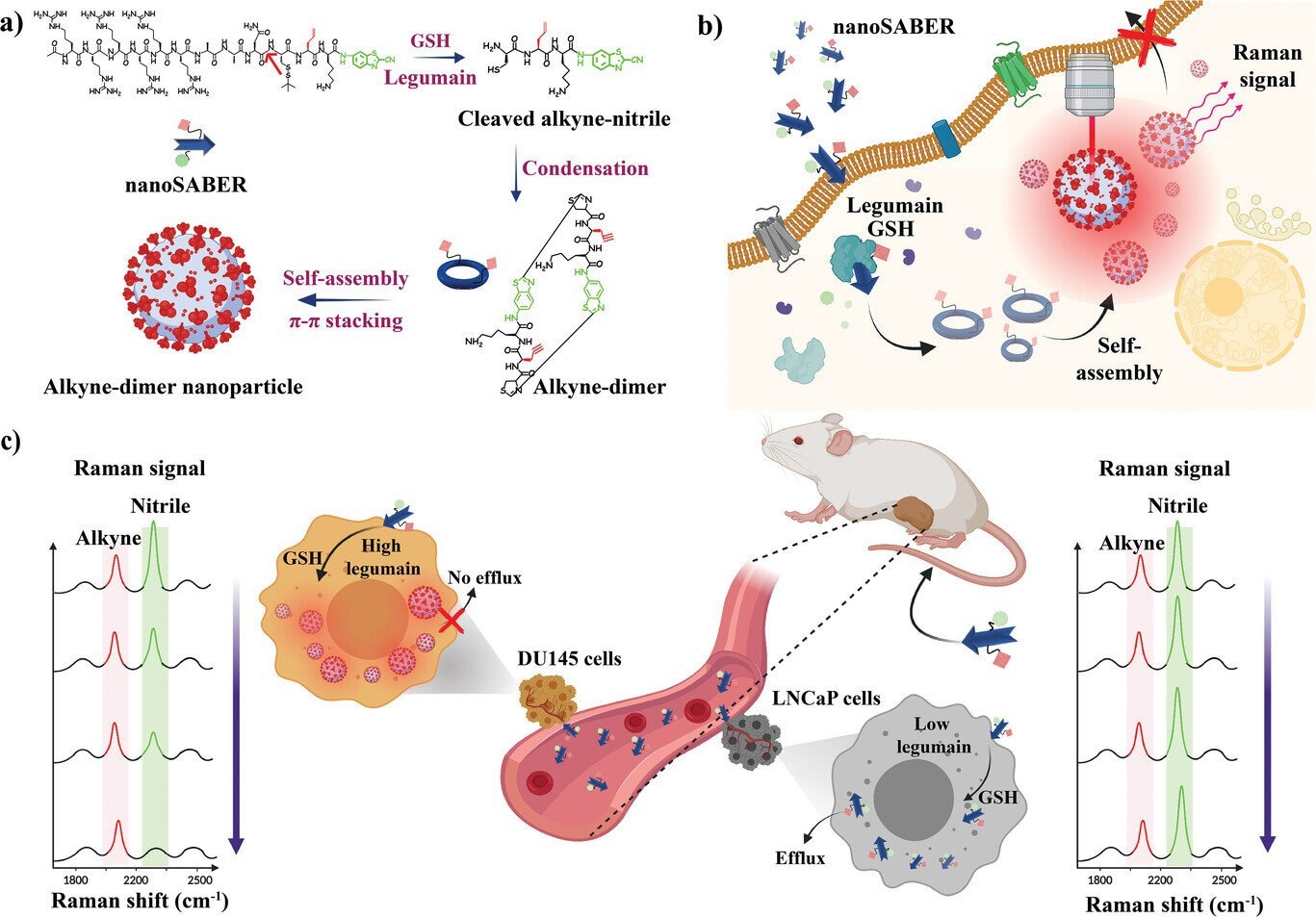
Schematic illustration for the formation of an alkyne-dimer nanoparticle by legumain-mediated intracellular reduction and condensation of the nanoSABER probe. a) Sequence of reaction steps showing the transformation of nanoSABER into a supramolecular self-assembled structure. Red arrow shows the site of legumain cleavage, with the alkyne and nitrile Raman reporters shown in red and green, respectively. b) After intracellular internalization of nanoSABER in high legumain-expressing cells (DU145 cells), it undergoes reduction by GSH and cleavage by the legumain enzyme. Alkyne-dimers are then formed, followed by self-assembly into alkyne-dimer nanoparticles as a result of π–π stacking. c) Schematic illustrating the utilization of nanoSABER for targeted Raman imaging of legumain activity in DU145 and LNCaP tumor-bearing mice. The spectral features associated with the nanoSABER probe were predominantly detected within the DU145 tumors because of legumain enzyme-triggered intracellular self-assembly, which also resulted in a prolonged probe retention time. Credit: Advanced Science (2023). DOI: 10.1002/advs.202304164
Taking inspiration from the process by which proteins are assembled in cells, this cutting-edge technology, spearheaded by Ishan Barman of the university's Whiting School of Engineering and Jeff W. Bulte, a Distinguished Professor of Radiology and Radiological Science at the School of Medicine, introduces infinitesimal nanoSABER probes that emit detectable signals upon encountering target enzymes within cancer cells.
The implications of this innovation are profound, promising enhanced cancer imaging capabilities, informed treatment decisions, and improved patient outcomes.
Revolutionizing Cancer Imaging
The potential applications of nanoSABERs in various industries are as diverse as they are promising. Here are some key ways this technology could reshape the landscape:
-
Precision Medicine: The ability to visualize tumors comprehensively, down to the molecular and cellular levels, represents a pivotal advancement in precision medicine. Tailoring treatments based on the real-time status of the tumor is an exciting prospect. The team's approach could help to reduce treatment side effects and maximize therapeutic impact.
-
Early Detection: The gold standard for cancer detection has long been tissue biopsies, which, while effective, are not without limitations. NanoSABER probes could potentially facilitate earlier and more accurate detection of cancerous activity. This promises to be a game-changer in early intervention and treatment.
-
Treatment Efficacy Monitoring: The real-time monitoring of drug accumulation within tumors during treatment is a valuable asset. By offering insights into the effectiveness of cancer therapies, nanoSABER technology may aid clinicians in optimizing treatment strategies, reducing recurrence rates, and improving overall patient outcomes.
-
Biopharmaceutical Development: The pharmaceutical industry stands to gain from nanoSABER technology by employing these probes to assess the effectiveness of cancer drugs in pre-clinical and clinical studies. This may streamline the drug development process and lead to more targeted therapies.
-
Biotechnology and Diagnostics: NanoSABER probes could have a significant impact on the development of diagnostic tools for various cancers. By offering a clearer picture of the molecular and cellular intricacies of tumors, this technology has the potential to improve the accuracy of diagnostic tests and enhance our understanding of cancer biology.
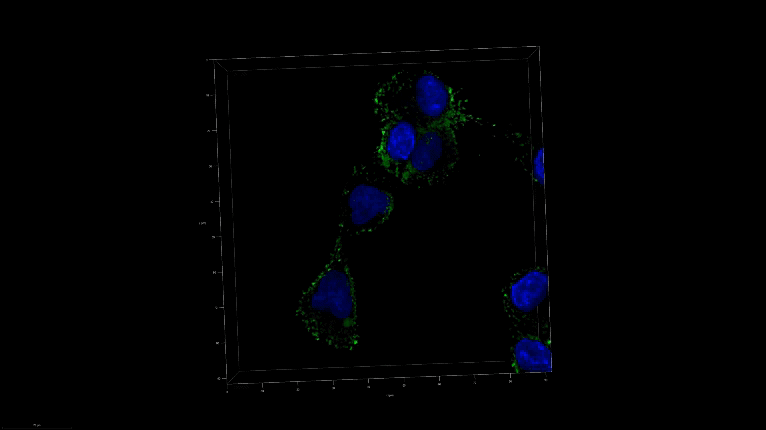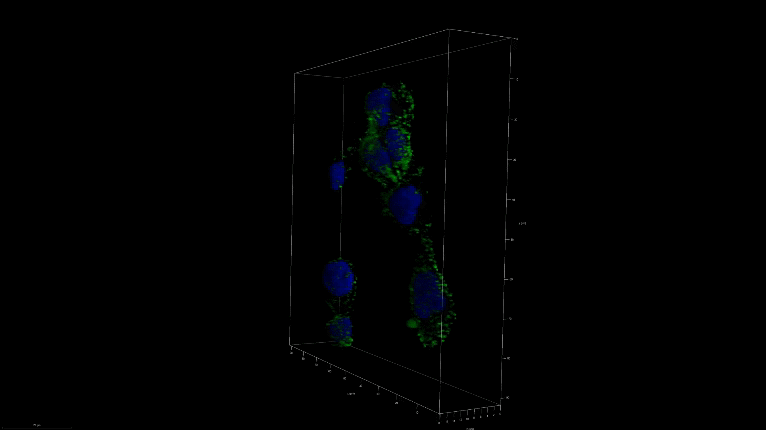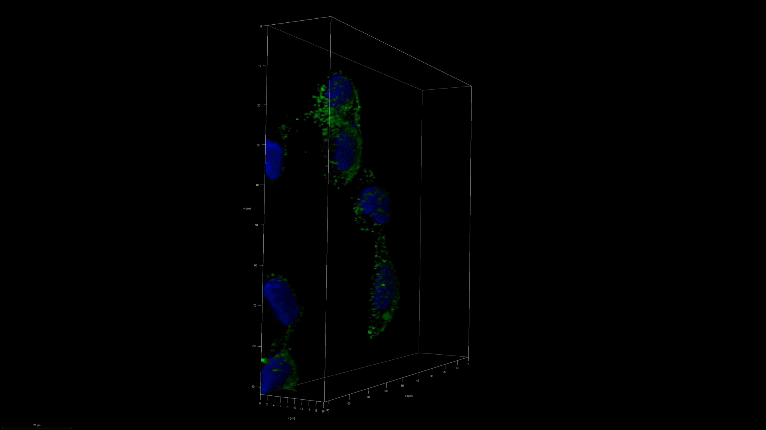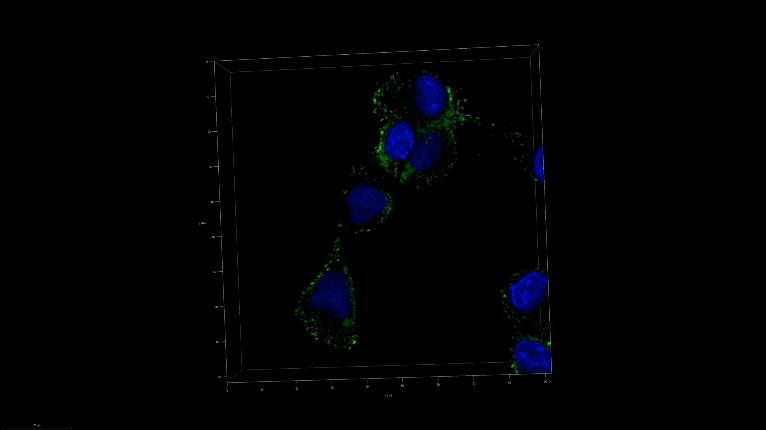
A 3D image of nanoSABER in DU145 cells. Credit: Johns Hopkins University
The NanoSABER Technology
The nanoSABER technology is based on a self-assembling biorthogonal enzyme recognition mechanism. These probes respond specifically to certain cancer-related enzymes, with a particular focus on legumain, a key player in cancer development and progression. When in the presence of these enzymes, the probes spontaneously form unique supramolecular structures, producing distinct vibrational signatures in the cell-silent Raman window.
This distinct signal, generated through Raman spectroscopy, provides an accurate and specific way to pinpoint the presence of cancer cells. This novel approach has the potential to overcome the limitations of current cancer imaging techniques, which may miss vital details lurking in the margins of tumors.
The Promise of Comprehensive Imaging
Lead Author Swati Tanwar, a post-doctoral fellow in mechanical engineering at Johns Hopkins, emphasizes the need for a comprehensive perspective on tumor behavior. Understanding the intricacies of tumor margins is vital for complete cancer removal and the prevention of recurrences. NanoSABER technology delivers on this front by offering a clear view at molecular, cellular, and tissue levels.
The team at Johns Hopkins, including co-authors Behnaz Ghaemi, Piyush Raj, Aruna Singh, Lintong Wu, Dian R. Arifin, and Michael T. McMahon, alongside Yue Yuan from the University of Science and Technology of China, has demonstrated the versatility and potential of the nanoSABER probe for Raman-based recognition of tumor aggressiveness, drug accumulation, and treatment response.
In summary, the introduction of nanoSABER technology has the potential to transform cancer imaging, early detection, and treatment.
By offering precise insights into tumor behavior at multiple levels, this breakthrough paves the way for a new era of cancer care and research. The applications are wide-ranging, from precision medicine and biopharmaceutical development to biotechnology and diagnostics, promising advancements that can have a lasting impact on the battle against cancer. As this pioneering technology continues to evolve, its impact on the field of oncology is poised to be nothing short of revolutionary.
Reference
Swati Tanwar et al (2023) A Smart Intracellular Self‐Assembling Bioorthogonal Raman Active Nanoprobe for Targeted Tumor Imaging, Advanced Science (2023). DOI: 10.1002/advs.202304164