A team of researchers from the Institute for Optoelectronic Systems and Microtechnology at Universidad Politécnica de Madrid (UPM) have designed a biosensor capable of identifying proteins and peptides in quantities as low as a single monolayer. For that, a surface acoustic wave (SAW), a kind of electrically controlled nano earthquake on a chip, is generated with an integrated transducer to act on a stack of 2D materials coated with the biomolecules to be detected.
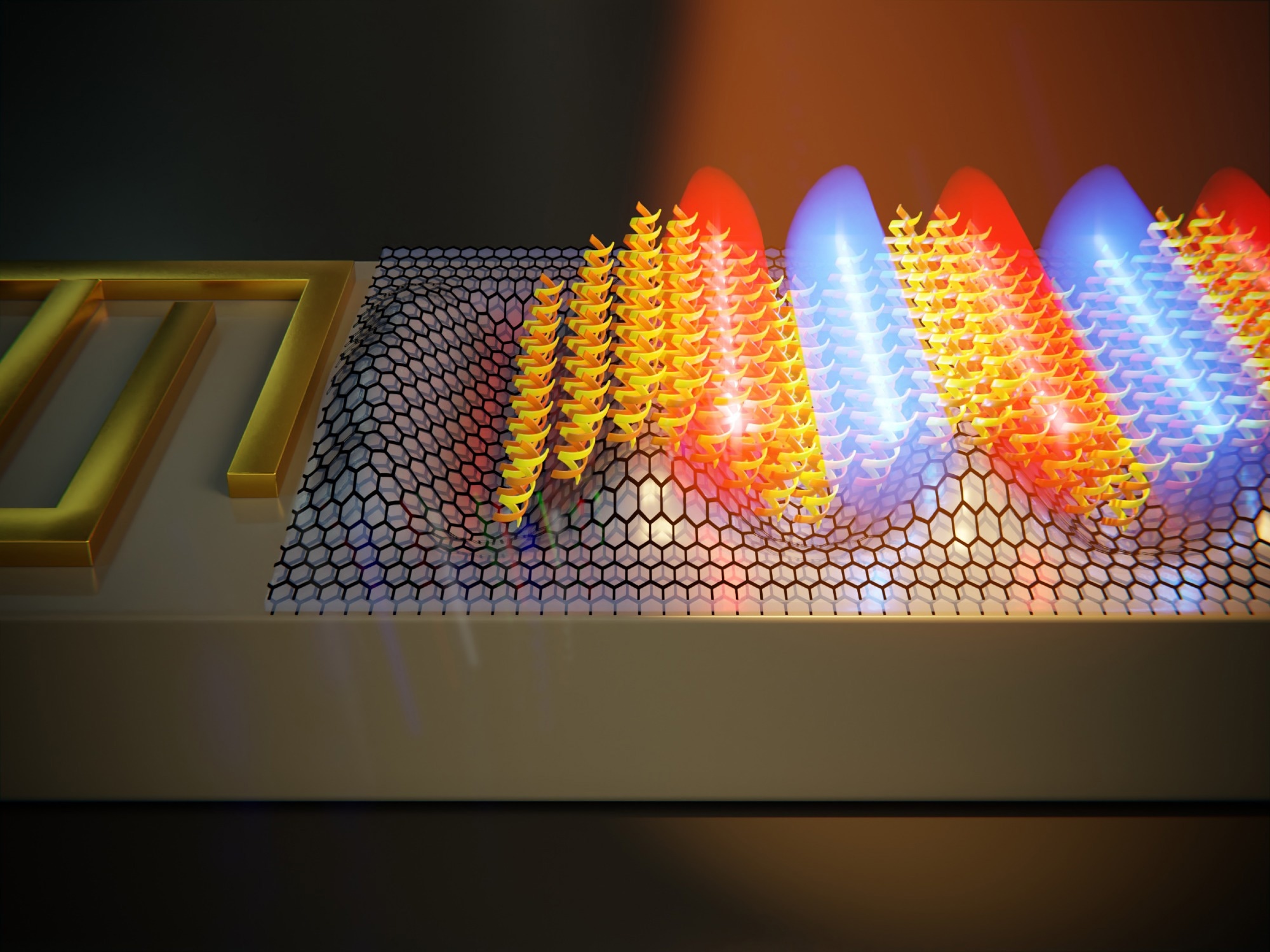 A surface acoustic wave launched by an interdigital transducer ripples the surface of the biosensor, confining light at the nanoscale to make it interact more efficiently with the molecules (Image: Jorge Pedrós, Raúl Izquierdo (UPM) and Enrique Sahagún (Scixel)).
A surface acoustic wave launched by an interdigital transducer ripples the surface of the biosensor, confining light at the nanoscale to make it interact more efficiently with the molecules (Image: Jorge Pedrós, Raúl Izquierdo (UPM) and Enrique Sahagún (Scixel)).
As they report in the journal Biosensors and Bioelectronics (Surface-acoustic-wave-driven graphene plasmonic sensor for fingerprinting ultrathin biolayers down to the monolayer limit), the SAW would ripple the surface of a graphene-based stack, in such a way that it confines mid infrared light to very small volumes, enhancing light-matter interactions at the nanoscale. In particular, quasiparticles that are part light (photons) and part matter (electrons and lattice vibrations), called surface plasmon-phonon polaritons, are formed at the rippled stack interplaying strongly with the molecules atop.
Organic molecules absorb certain wavelengths of light in the mid infrared range that are characteristic of their chemical composition and structure. Therefore, this set of absorption resonances, called their vibrational fingerprint, allows for the identification of the organic compound. “By strengthening the interaction between light and biomolecules deposited on top of the sensor, we would be able to identify analytes requiring smaller quantities, reaching levels as low as a single monolayer”, says Raúl Izquierdo, first author of this study.
According to Jorge Pedrós, leading scientist of the study, “One advantage of thismechanism is that SAWs are actively controlled through a high frequency voltage,allowing to easily switch between an ON configuration, at which interaction isincreased, and an OFF configuration, without any improvement to the signal. This measuring scheme increases the sensor resolution”.
In addition to the design of the sensor and the calculations of its performance, theauthors also provide a mathematical method to extract apparently hidden quantitative information, further increasing the sensitivity of the sensor. For that, the molecules of the analyte and the surface plasmon-phonon polaritons are modelled as oscillators that interact with each other, while both are driven by an external force (light incident on the sensor). Despite its simplicity, this model is shown to reproduce nicely the results from the calculations.
To conclude, the authors are confident that this study will contribute to the development of new lab-on-chip devices, combining the chemical fingerprinting capability of this novel SAW-driven biosensor with other acoustic functionalities such as SAW-based mass sensing or droplet streaming and mixing in microfluidiccircuits.