According to Penn State researchers, a newly created “GPS nanoparticle” can be administered intravenously and target cancer cells to deliver a genetic punch to the protein involved in tumor development and dissemination.
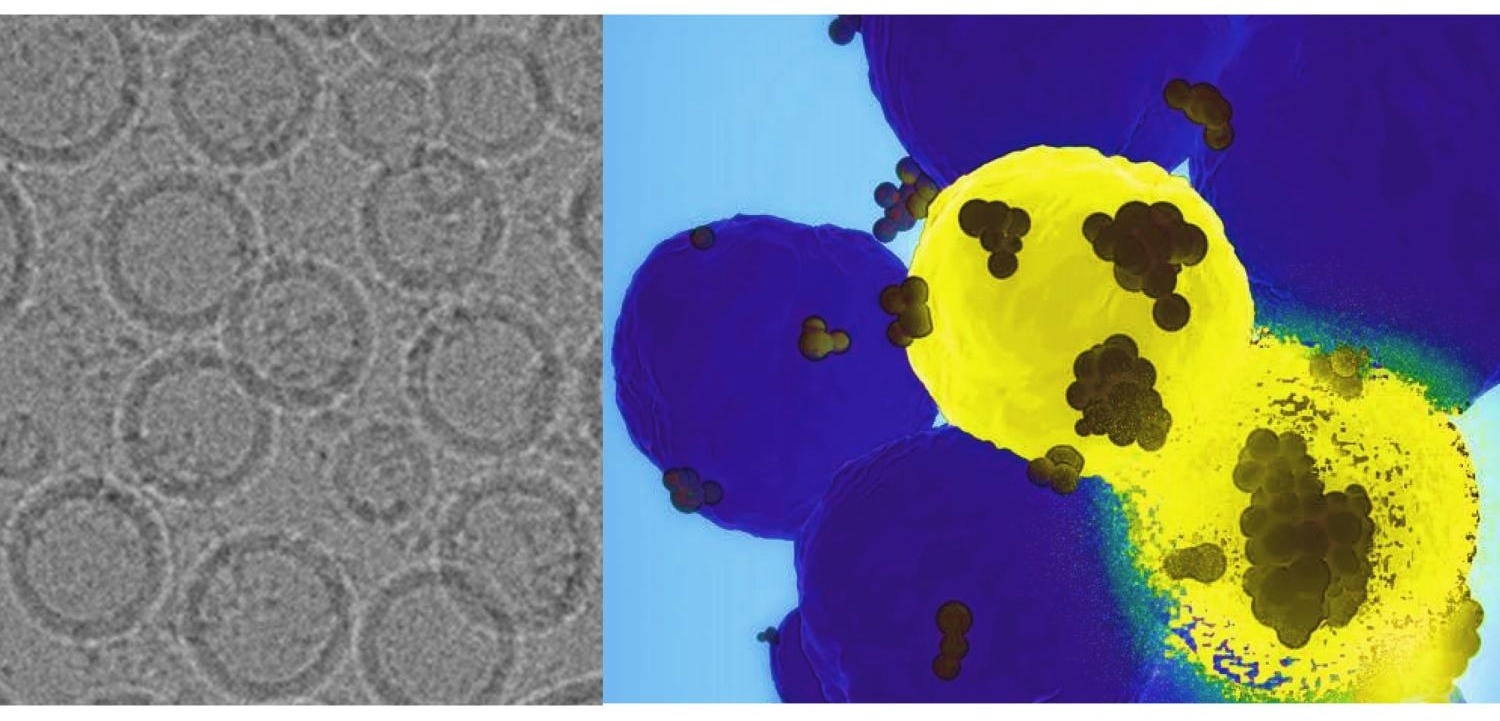 On the left is an electron microscopic image of the nanoparticles designed by the team led by Penn State researchers. On the right, the nanoparticles, shown as black dots, find the basal-like breast cancer cells in yellow and deliver the gene-editing tools. The disintegrating cell demonstrates the destructive power of the therapy delivered by the nanoparticle. Image Credit: Provided by Dipanjan Pan
On the left is an electron microscopic image of the nanoparticles designed by the team led by Penn State researchers. On the right, the nanoparticles, shown as black dots, find the basal-like breast cancer cells in yellow and deliver the gene-editing tools. The disintegrating cell demonstrates the destructive power of the therapy delivered by the nanoparticle. Image Credit: Provided by Dipanjan Pan
Their strategy to successfully knock down a cancer-causing gene was evaluated in mice and human cell lines. They reported that the technology could offer a more accurate and successful treatment for basal-like breast tumors, which are known to be extremely difficult to cure.
On March 11th, 2024, their study was published in ACS Nano. Additionally, they submitted a provisional patent application for the technique included in this study.
We developed a GPS nanoparticle that can find the site where it is needed. Once there — and only there — it can deliver gene editing proteins to prevent the cancer cells from spreading. It was a difficult task, but we showed that the system works for basal-like breast cancers.
Dipanjan Pan, Dorothy Foehr Huck & J. Lloyd Huck Chair Professor in Nanomedicine, Pennsylvania State University
Though less common than other breast cancers, basal-like tumors can be much harder to treat due to their lack of the three therapeutic targets present in other breast cancer types, which is similar to triple-negative breast cancers.
They also usually develop rapidly, shedding cells that travel to other parts of the body and becoming aggressive tumors. This procedure, known as metastasis, allows those cells to spawn new cancers.
Pan added, “Metastasis is a huge challenge, especially with cancers like triple-negative and basal-like breast cancers. The cancer can be hard to detect and does not show up during a routine mammogram, and it primarily affects the younger or African American population who may not be receiving preventative care yet. The outcome can be very, very poor, so there is a clear unmet clinical need for more effective treatments when the cancer isn’t caught early enough.”
The scientists created a Trojan horse nanoparticle by covering it with specially designed fatty molecules that resemble naturally occurring lipids and filling it with CRISPR-Cas9 molecules. These molecules can target a cell’s genetic material, identify a certain gene, and either knock it down or render it useless. In this scenario, the system targeted human forkhead box c1 (FOXC1), which is responsible for metastasis.
Pan classified the designer lipids as “zwitterionic,” which means they have a near-neutral charge on the nanoparticle shell. This stops the body’s immune system from fighting the nanoparticle since it is camouflaged as a non-threatening, normal molecule, and it can help release the payload if the lipids detect the cancer cell’s low pH environment.
To guarantee that the lipids only activated at that low pH, the researchers engineered them to charge positively once they entered the more acidic tumor microenvironment, prompting payload release.
However, given the size of the body, how could the researchers be certain that the CRISPR-Cas9 payload reached the intended location? Additionally, scientists appended an epithelial cell adhesion molecule (EpCAM), which is known to connect to basal-like breast cancer cells, to make sure the nanoparticle would link to the proper cells.
“No one has ever attempted to target a basal-like breast-like cancer cell with context-responsive delivery system that can genetically knockdown the gene of interest. We are the first to show that it can be done,” Pan noted.
Some researchers have created non-viral delivery methods that use nanoparticles as well as viral delivery systems that use a virus particle to deliver drugs to cells. The surface lipid in Pan’s team’s method, he claimed, is unique in that it reacts only in the target environment, minimizing the possibility of off-target delivery and damage to healthy cells.
Furthermore, he stated that there is less likelihood of an immune response since the body does not view lipids as a threat, a point that their studies supported.
To confirm that the nanoparticle would release the CRISPR/Cas9 system in the appropriate setting, the researchers initially tested the method on human triple-negative breast cancer cells. In a mouse model, they verified that the nanoparticle could locate a tumor, activate the mechanism, and effectively destroy FOXC1.
Pan stated that the researchers intend to test the nanoparticle platform further in the future with the ultimate objective of using it in human clinical settings.
Pan stated, “We are also exploring how else we might apply the platform technology. We can customize the molecules on the surface, the payload it carry, and use it to encourage healing in other areas. There is a lot of potential with this platform.”
David Skrodzki, Matthew Molinaro, Nivetha Gunaseelan, all doctoral students at Penn State; Dinabandhu Sar, University of Illinois, Urbana-Champaign; Teresa Aditya, postdoctoral researcher in nuclear engineering at Penn State; Dipendra Dahal and Priyanka Ray, both postdoctoral researchers in Pan’s laboratory at his previous institution of the University of Maryland Baltimore are the other authors.
This study was supported by Penn State, the University of Maryland Baltimore School of Medicine, the Centers for Disease Control and Prevention, the U.S. National Science Foundation, and the US Department of Defense Congressionally Directed Medical Research Program.
Journal Reference:
Moitra, P., et. al. (2024) Context-Responsive Nanoparticle Derived from Synthetic Zwitterionic Ionizable Phospholipids in Targeted CRISPR/Cas9 Therapy for Basal-like Breast Cancer. ACS Nano. doi:10.1021/acsnano.4c01400.