Mar 14 2008
Ions—charged atoms or molecules—play an important role in nature, in our bodies as well as for science and technology. It is often necessary to trap, remove, mask, stabilize, or transport ions, whether in the body or the lab. With positively charged metal ions, this goal is often achieved with chelate ligands, organic molecules that tightly grab hold of the ions. However, it is difficult to develop suitable chelators for negatively charged anions such as chloride and fluoride. Amar Flood and Yongjun Li at Indiana University (Bloomington, USA) have now synthesized a donut-shaped molecule that tightly and selectively takes chloride ions up into its center. As they report in the journal Angewandte Chemie , bridging hydrogen bonds are responsible for holding the chloride ion in place.
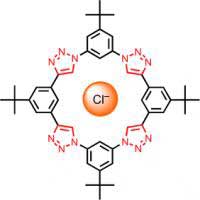 When traditionally weak C-H hydrogen bonds are preorganized in a shape-persistent macrocycle, a strong affinity for chloride ions emerges. This discovery arises from the dual use of click chemistry for efficient macrocyclization and the favorable steric properties of the resulting 1,2,3-triazoles. Credit: (C) Wiley-VCH 2008 http://dx.doi.org/10.1002/anie.200704717
When traditionally weak C-H hydrogen bonds are preorganized in a shape-persistent macrocycle, a strong affinity for chloride ions emerges. This discovery arises from the dual use of click chemistry for efficient macrocyclization and the favorable steric properties of the resulting 1,2,3-triazoles. Credit: (C) Wiley-VCH 2008 http://dx.doi.org/10.1002/anie.200704717
Chelators (from the Greek word for pincer) are small organic molecules that grab onto atoms or other small molecules, holding them by means of multiple binding sites. Chelate therapy is used to absorb and remove heavy metals in cases of poisoning, for example. It is a breeze to bind cations in this way. The development of organic molecules whose positively charged “arms” are arranged so as to tightly and selectively bind anions has not been successful to date.
Flood and Li found their new anion chelator more or less by coincidence when they were producing various macrocycles by means of an inexpensive, flexible synthetic technique known as “click chemistry”, which is a simple and efficient way to put molecules together into large entities. The researchers “clicked” four small rings together to form a large ring. This process also generates four more rings, made of three nitrogen atoms and two carbon atoms (triazole rings). These are not only by-products of the click chemistry, they are essential for binding the chloride ion, which can comfortably nestle into the empty center of the large donut-shaped ring. The triazoles hold on to the chloride ion by means of bridging hydrogen bonds, which is amazing because it was previously assumed that hydrogen bonds were not strong enough to form a sufficiently stable bond between a halogen ion and a chelate complex. It is probably vital that the binding sites in the structurally stable macrocycle are preorganized into the correct configuration so that the chelator does not have to rearrange itself around the ion before binding can occur, as is the case for open-chain chelators.
The four other nonbinding rings of the macrocycle can be varied almost as desired, so the researchers hope to generate a whole family of new chelators that are able to bind a spectrum of other anions with high specificity.