NanoViricides, Inc., said that an internationally renowned Japanese ophthalmologist and corneal researcher, Kazuo Tsubota, MD, PhD, has agreed to perform confirmatory animal efficacy studies of the nanoviricide anti-EKC drug candidate, EKC-Cide(TM), against EKC (epidemic kerato-conjunctivitis) in Japan. Dr. Tsubota is currently Professor and Chairman of the Department of Ophthalmology at Keio University School of Medicine in Tokyo, Japan.
“NanoViricides’ study of clinical efficacy of EkcCide, is very significant. Their study conclusively demonstrated clearance of the severe pink eye symptoms,” said Dr. Tsubota, after a discussion with the Company scientists in a meeting in Boston last week. He commented, “EKC and herpes virus infections of the eye are important medical problems in Japan, and Japanese pharmaceutical companies have a strong interest in potential treatments,” He continued by saying, “I am very pleased to have the opportunity to confirm the studies in Japan. This is the first step towards obtaining Japanese regulatory approval.”
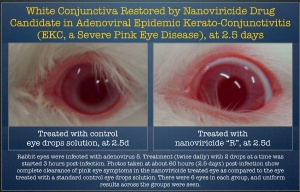
EKC is a severe and contagious viral infection of the eye in humans which can lead to severe visual impairment. Viral EKC is primarily caused by adenoviruses, although some other viruses may also be the causative agent in humans. There is no currently available treatment for viral EKC.
The Company has previously reported that EkcCide was shown to cause rapid and clear clinical recovery of infected animal eyes in a study conducted by a renowned US ophthalmologic Institute. The infection in this study was caused by adenovirus 5, supplied by the Centers for Disease Control and Prevention (CDC). Representative photographs are posted on the Company’s website.
The Company anticipates that the eye-drop formulation of the broad-spectrum nanoviricide drug candidate which was successful against adenoviral EKC may have significant potential against other viral EKC and the less severe viral conjunctivitis diseases as well. Common viral conjunctivitis is highly contagious and occurs primarily in schools and other crowded settings, as well as in immuno-compromised populations. While the Company currently has no approved product for the treatment of EKC and viral conjunctivitis, the treatment and prophylaxis market for EKC and other viral causes of conjunctivitis is expected to be in the order of several billion dollars annually.