Aug 5 2008
Scientists from Germany, Canada and the Netherlands have studied tiny gold nanoparticles, so-called clusters, and found them to have fascinating arrangements of their constituent atoms. For example, twenty gold atoms form a tetrahedron, a sort of pyramid. The nineteen-atom cluster is a truncated pyramid, which can be formed by cutting off one corner atom from the twenty-atom gold pyramid. The structures have been identified using the Free Electron Laser for Infrared eXperiments FELIX at the FOM-Institute for Plasma Physics Rijnhuizen in Nieuwegein. Detailed knowledge about the geometries of such nanoparticles can lead to a better understanding of the unexpected catalytic activity of very small gold particles. The researchers published their results in Science on 1 august 2008.
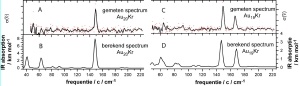 Vibrational spectrum of gold nanoparticles The left panel shows the experimental (A) and calculated (B) vibrational spectrum of a cluster with twenty gold atoms. The gold atoms form a highly symmetric structure: a triangular pyramid, a tetrahedron that has four equivalent corners and faces. The high degree of symmetry gives rise to a very simple spectrum: only a single intense feature is observed. In the right panel the experimental (C) and calculated (D) spectrum of a gold cluster with nineteen gold atoms is shown. This cluster exhibits reduced symmetry. It still forms a pyramid but missing on of the corner atoms. Correspondingly the drop of symmetry is reflected in a more complex infrared spectrum consisting of two lines. Very good agreement is observed between experiment and theory. Here, Kr stands for Krypton which functions in the experiment as messenger but has no influence on the structure.
Vibrational spectrum of gold nanoparticles The left panel shows the experimental (A) and calculated (B) vibrational spectrum of a cluster with twenty gold atoms. The gold atoms form a highly symmetric structure: a triangular pyramid, a tetrahedron that has four equivalent corners and faces. The high degree of symmetry gives rise to a very simple spectrum: only a single intense feature is observed. In the right panel the experimental (C) and calculated (D) spectrum of a gold cluster with nineteen gold atoms is shown. This cluster exhibits reduced symmetry. It still forms a pyramid but missing on of the corner atoms. Correspondingly the drop of symmetry is reflected in a more complex infrared spectrum consisting of two lines. Very good agreement is observed between experiment and theory. Here, Kr stands for Krypton which functions in the experiment as messenger but has no influence on the structure.
Gold is known to be a noble metal which means that it is inert and shows little reactivity. The resistance of gold to corrosion has been known to the human for millennia and is reflected in the use of gold in jewelry and currency. For the chemist, however, the reluctance of gold to undergo reactions was a reason to show little interest in this metal once the days of alchemy were past. This changed a few years ago when it was found that very small gold particles can catalyze, i.e. accelerate, important chemical reactions, for example the oxidation of hydrogen and carbon monoxide. Reasons for the high reactivity of these nanoscopic particles, so-called clusters, are assumed to be hidden in their atomic structure. As the active nanoparticles are smaller than the wavelength of visible light their structure cannot be inspected using an optical microscope. In the recent past, the structure of charged gold clusters had been determined, but that of the neutral clusters, thought to be more relevant for the catalytic activity, remained elusive.
Now, an international collaboration of scientists from the FOM-Institute for Plasma Physics Rijnhuizen in Nieuwegein, the Fritz-Haber-Institut der Max-Planck-Gesellschaft in Berlijn and the Steacie Institute for Molecular Sciences in Ottawa, Canada, has succeeded in identifying the structures of neutral gold clusters. They observed how the gold clusters interact with infrared laser light from the free-electron laser FELIX at the FOM-Institute for Plasma Physics in Nieuwegein.
The scientists produced the gold clusters by evaporating them from solid gold metal and subsequently using a helium gas beam to pick them up and to cool the particles. The cold beam of gold clusters contains cluster sizes from three to twenty atoms. The clusters are exposed to the intense infrared light. The scientists investigate the response to different frequencies of the infrared light. The atoms in molecules and clusters are held together by chemical bonds, which can be thought of as springs. If the frequency of the infrared light is matched to the frequency of the spring the light will amplify this vibration. In the very intense light of FELIX the spring can start to vibrate so strongly that the particle falls apart. Using a mass spectrometer the scientists can measure this process and reconstruct the vibrational spectrum.
The structure of the gold clusters can be determined by comparing the vibrational spectrum with those predicted using quantum chemistry theory. The complexity of the vibrational spectrum relates strongly to the symmetry of the cluster. Highly symmetric clusters, i.e. clusters which appear to be the same when observed from different sides, exhibit simple spectra with few but often intense signals. For example, only a single intense feature was observed in a cluster that contained precisely twenty gold atoms. This is the finger print of a highly symmetric structure that is shown to be a triangular pyramid, a tetrahedron that has four equivalent corners and faces. The spectrum of the cluster with nineteen gold atoms is more complex, reflecting a drop in symmetry. Analysis of the vibrational spectrum shows that the structure is that of the twenty atom cluster with a gold atom missing from one of the corners. The neutral clusters with nineteen and twenty atoms show the same structures that are familiar from their negative counterparts. A neutral cluster with seven gold atoms shows an even more complicated spectrum that matches a two-dimensional, asymmetric structure where six atoms form a triangle with one additional gold atom attached. In contrast to the larger clusters, for gold-seven the positively charged cluster arranges differently. In this cluster six atoms form a hexagon with one atom in its centre.
A new way is opened to study a whole range of gold clusters and clusters of other catalytically important metals at the molecular level. Once their structures are revealed the same techniques can be applied to follow intermediate steps of catalytic reactions on nanoparticles.