Sep 12 2008
Researchers in Japan have revealed important information about why the threshold of gas pressure required for the structural transformation of flexible, three-dimensional molecular networks known as porous coordination polymers (PCPs) varies for different gases. The ability of PCPs to reversibly change their structure and properties on adsorption of 'guest' gas molecules has received considerable attention in recent years owing to their commercial potential in gas separation and sensing applications.
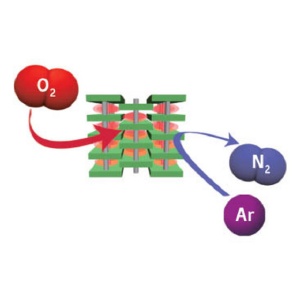 Selective gas adsorption of flexible PCPs. The onset pressure for the gate-opening process is lower for oxygen (O2) than for nitrogen (N2) and argon (Ar). As a result, within a certain pressure range, only oxygen adsorbs.
Selective gas adsorption of flexible PCPs. The onset pressure for the gate-opening process is lower for oxygen (O2) than for nitrogen (N2) and argon (Ar). As a result, within a certain pressure range, only oxygen adsorbs.
Flexible PCPs exist in two forms: a closed phase, in which guests, such as oxygen molecules, are unable to penetrate the structure; and an adsorbed phase that allows sorption of oxygen into the structure. Transformation from the closed to the adsorbed phase, which results in opening the ‘gates’ to structural channels, is guest-induced and occurs when the pressure of the gas—defined as Pgo—is sufficient.
The research team, led by Susumu Kitagawa and including researchers from the RIKEN SPring-8 Center in Harima and from Kyoto, Okayama and Osaka-Prefecture universities, has used a kinetic study to show why the value of the threshold pressure required for the initially closed structure to become accessible is different for similar gases*. Much of the commercial interest sparked in these materials has arisen from the difference of sorption behavior, that is, the difference in Pgo for similar gases.
The flexible PCP synthesized by the team is formed from cadmium ions and organic ligands and shows that the value of Pgo increases in the order of oxygen, argon and nitrogen and so, at low pressures, only oxygen is adsorbed.
The researchers believe that their kinetic investigation of the structural transformation should further our knowledge and hence, allow the gas adsorption performance of PCPs to be fine-tuned.
According to RIKEN team-member Masakazu Higuchi, an ‘intermediate phase’ exists at the point of gate-opening and, it is the formation kinetics of this intermediate that determines Pgo. Moreover, the kinetic behavior suggests that the gate-opening process is attributable to condensation of the adsorbate on the surface of the crystal, indicating that surface chemistry, rather than crystal structure analysis, needs to be probed to fully understand the gate-opening process.
The team hopes to investigate the process further by observing the surface using microscopic techniques and, in the long-term future, it may be possible to modify the crystal surface and hence, create a new mechanism for adsorption.
- Tanaka, D., Nakagawa, K., Higuchi, M., Horike, S., Kubota, Y., Kobayashi, T. C., Takata, M. & Kitagawa, S. Kinetic gate-opening process in a flexible porous coordination polymer. Angewandte Chemie International Edition 47, 3914–3918 (2008).