Oct 24 2008
Some organic substances have a property called photochromism, meaning that their absorption spectrum, or color, changes when they are exposed to certain types of light. In particular, a new artificial protein called Dronpa shows great promise for applications because it can be switched back and forth between a 'bright' state and a 'dark' state. Now Atsushi Miyawaki, Hideaki Mizuno at the RIKEN Brain Science Institute in Wako and co-workers* have explained for the first time exactly what causes Dronpa to move between these two states.
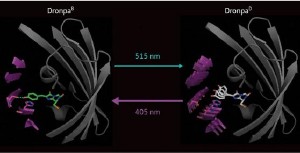 Comparison of the bright (DronpaB) and dark (DronpaD) states of the Dronpa protein. In the bright state, the chromophore (green) is tethered to the molecule by a hydrogen bond (dotted blue line), while in the dark state the hydrogen bond is gone and the chromophore can vibrate.
Comparison of the bright (DronpaB) and dark (DronpaD) states of the Dronpa protein. In the bright state, the chromophore (green) is tethered to the molecule by a hydrogen bond (dotted blue line), while in the dark state the hydrogen bond is gone and the chromophore can vibrate.
Dronpa was developed by Miyawaki and colleagues by genetic engineering on a wild coral protein. Usually Dronpa absorbs light of around 503 nanometers wavelength and emits green fluorescence-the so-called bright state. However if it is exposed to strong radiation at 488 nanometers it converts into the dark state, which emits no fluorescence. The protein will switch back to the bright state if it is re-irradiated at an even shorter wavelength.
"Such reliable photochromism for Dronpa prompted us to develop it for information storage with the ability to record, erase, or read information" says Miyawaki. However to date no-one has proven exactly what causes the photochromism.
To unlock this mystery the researchers used nuclear magnetic resonance (NMR) spectroscopy to study the Dronpa molecule, which takes a cylindrical shape called a ß-barrel, like other fluorescent proteins. They discovered that the bright and dark states arise from interactions between the ß-barrel and the chromophore-the part of the molecule responsible for light absorption.
In the bright state, the chromophore is tightly tethered to the ß-barrel by a hydrogen bond. This holds the chromophore in a rigid, flat configuration, so that when it is excited by light it releases its excess energy by fluorescing.
When the dark state is induced, the hydrogen bond is lost and the chromophore becomes much more flexible. Therefore it releases the excess energy by vibrating instead of fluorescing.
In other compounds that have been studied, photochromism results from physical rearrangements of atoms in the molecules. This is the first time that photochromism has been linked to structural flexibility.
"We present a new molecular mechanism for photochromism of a fluorescent protein" says Miyawaki. "The mechanism requires a special microenvironment involving a ß-barrel, a structure not present in organic photochromic compounds."
Miyawaki hopes that Dronpa could eventually be used in very high-resolution optical microscopy. "The next stage will be to develop many mutants of Dronpa with different photochromic properties" he says.
- Mizuno, H., Mal, K.T., Wälchli, M., Kikuchi, A., Fukano, T., Ando, R., Jeyakanthan, J., Taka, J., Shiro, Y., Ikura, M. & Miyawaki, A. Light-dependent regulation of structural flexibility in a photochromic fluorescent protein. Proceedings of the National Academy of Sciences USA 105, 9927-9932 (2008).