 By Isabelle Robinson, M.Sc.Feb 25 2019
By Isabelle Robinson, M.Sc.Feb 25 2019Buckminsterfullerene was first discovered in 1985 by a research team from Rice and Sussex University and was named after the American architect Buckminster Fuller due to its structural similarity to a geodesic dome he designed.
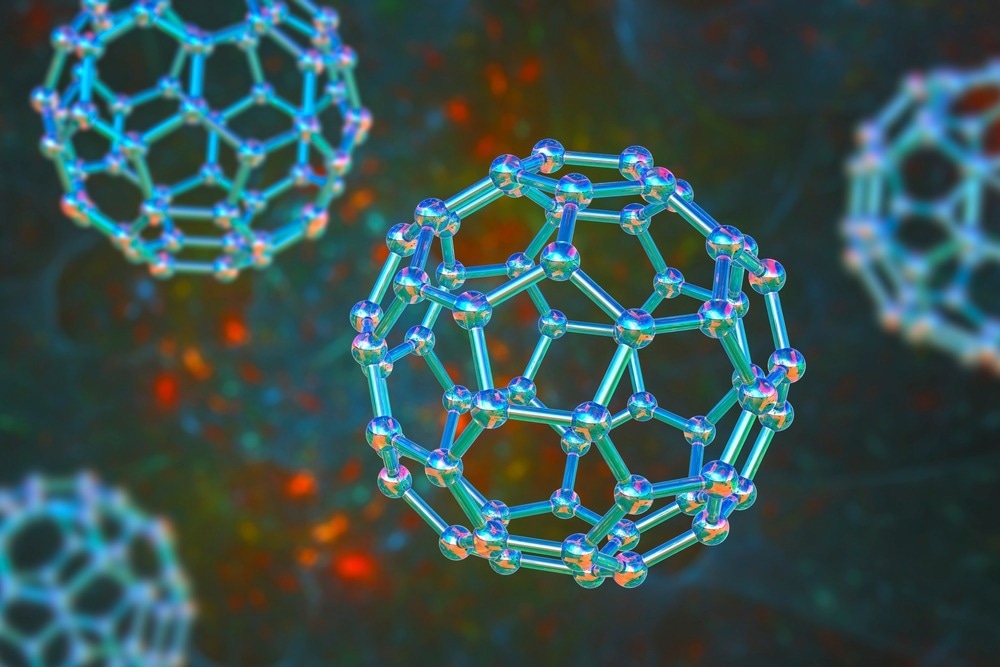
Image Credit: Kateryna Kon/Shutterstock.com
Buckminsterfullerene (C60) is an allotrope of fullerene and has a structure that resembles a soccer ball. Due to the Buckminsterfullerene's shape, where carbon atoms are arranged into 20 hexagons and 12 pentagons, it is sometimes colloquially referred to as “buckyballs”. This type of molecule is known as an icosahedron since it has 60 vertices and 62 faces.
The van der Waals range of the C60 molecule is approximately 1.01 nanometers with an average bond length of 0.14 nanometers. Carbon atoms in the fullerene are bonded covalently to 3 others. Buckminsterfullerene is simple to synthesize and has become a popular subject of materials research investigating the uses and properties of fullerenes, with a particular interest in its future applications.
Buckminsterfullerene's properties make it appealing to numerous industries. For example, as electrons are not delocalized, Buckminsterfullerene is not aromatic despite its carbon nature. More importantly, Buckminsterfullerene has a very high tensile strength and ductility.
Research has shown that this fullerene allotrope can maintain its original shape after being exposed to a 3000 atmospheric pressure. Because of these material properties, there have been many proposed applications of Buckminsterfullerene
Applications of Buckminsterfullerene
The most extensively used applications of Buckminsterfullerene have been in the biomedical field. Most notably, for its use as a potential antiviral agent and its ability to suppress the HIV virus, an immunodeficiency virus that can lead to AIDS.
Uses of this fullerene allotrope have also been included in the suppression of the hepatitis C virus as well as the vesicular stomatitis virus. It should also be noted that drug and gene delivery is also a medical sector in which Buckminsterfullerenes have been heavily researched.
Due to its ability to decrease the transmittance of light, Buckminsterfullerenes are able to be used as optical limiters. This means that they are particularly useful for developing protective eyewear and optical sensors.
Materials research has concluded that Buckminsterfullerene and other fullerenes will be the future of developing lightweight metals while achieving a greater tensile strength. According to researchers, this is due to the extremely small size of the carbon molecule and the comparably high reactivity due to the sp2 hybridization, which allows for a dispersion-strengthening metal matrix of fullerenes and metals. In a recent study, material developers were able to increase the hardness of the lightweight Ti-24.4AI-17N alloy by approximately 30% by adding fullerenes.
Furthermore, Argonne National Laboratories and MER Corporation have been able to demonstrate turning fullerene molecules into diamond molecules with a simple rearrangement of the atoms. This proves the similarity of the structure of fullerenes and diamonds, paving the way for an alternative substitute for diamond films needed in electronic devices.
In 2006, an Israeli company tested one of the most shock-resistant materials currently in use; the material is approximately five times stronger than conventional steel and at least twice as strong as the impact-resistant material in use at the time. The material was developed by ApNano and was tested by Prof. Yan Qiu Zhu of the School of Mechanical, Materials and Manufacturing Engineering at the University of Nottingham.
The materials contained fullerene and were subjected to steel projectiles traveling at 1.5km/second and were able to withstand shock pressures of approximately 250 tons per square centimeter. The fullerene-based material can be used as protective gear for soldiers as it is shock resistant and lightweight.
References and Further Reading
Ahmed, N. S. (2001, October 5). THE DOOR THAT THE BUCKYBALL OPENED. Retrieved from The Tech Online: https://www.mit.edu/
Boysen, E. (n.d.). Buckyballs: Uses and Applications | Fullerenes. Retrieved from UnderstandingNano: http://www.understandingnano.com/buckyballs-fullerenes.html
Iddo Genuth, T. Y. (2006, February 15). Protecting the soldiers of tomorrow. Retrieved from IsraCast: https://www.isracast.com/
Katz, E. A. (2006). "Fullerene Thin Films as Photovoltaic Material". In Sōga, Tetsuo. Nanostructured materials for solar energy conversion. Elsevier. pp. 361–443. ISBN 978-0-444-52844-5.
Ray, A. (2018, June 3). 8 Useful Applications of Fullerenes You'll Be Surprised to Know. Retrieved from ScienceStruck: https://sciencestruck.com/applications-of-fullerene
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.