
In this interview, AZoNano speaks with Professor Andre Nel about his involvement in innovative research describing the development of a 'glass bubble' nanocarrier that could help drug formulations access pancreatic cancer cells. Targeting cells within the pancreas is known to be challenging, but this new nanocarrier could help improve treatment for one of the most aggressive forms of cancer.
Please could you introduce yourself and tell us about your current position and research activities?
I am a distinguished Professor of Medicine in the David Geffen School of Medicine at UCLA, and I also serve as Research Director of the California NanoSystems Institute on the UCLA campus.
My research activities are based in nanomedicine and focus on developing custom designed nanocarriers for the treatment of cancer and diseases of the immune system using a variety of material types such as mesoporous silica, polymers, and lipid-based nanoparticles.
How did you become involved with this investigation in particular?
As a medical practitioner in the field of allergy and immunology, I launched several research centers to look into the role of air pollution particles in asthma, thereby introducing me to the world of nanoparticles and gaining awareness of the game-changing potential of nanotechnology at the start of the 21st century. This background allowed us to acquire a major national center to study the safe implementation of nanomaterials for the benefit of humans and the environment.
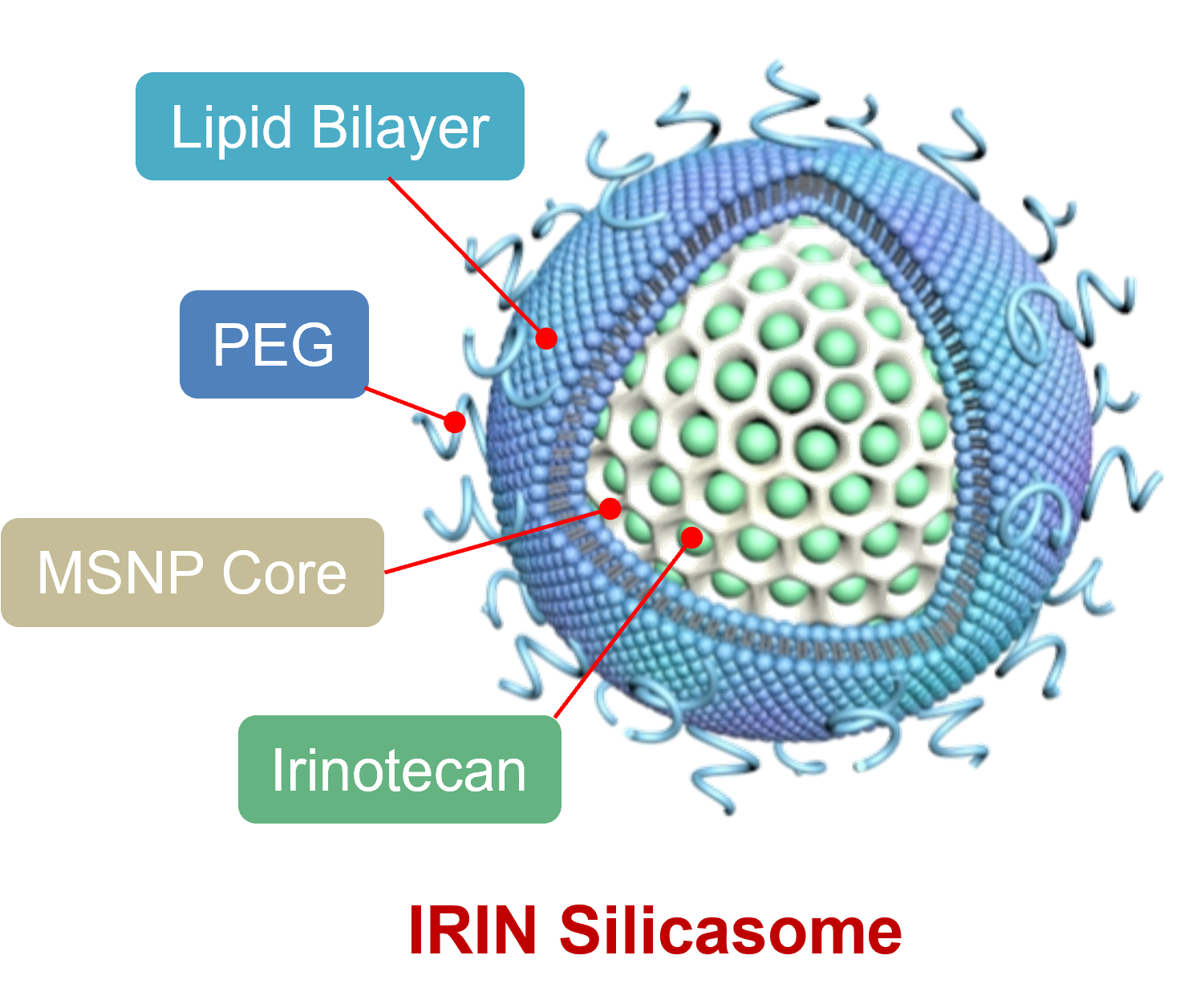
The silicasome carrier is comprised of a mesoporous silica interior, coated with a lipid bilayer on the outside. The chemo drug, in this case Irinotecan, is loaded into the porous interior by remote import through the lipid bilayer. The lipid bilayer serves to trap the drug and provide carrier safety. MSNP = mesoporous silica nanoparticles; PEG = polyethylene glycol (which serves to keep the drug carrier circulating for a long time to enable uptake at the pancreas cancer site.
Could you summarize the results of your recent research?
We developed a nanoparticle drug carrier platform that resembles hollow glass bubbles, also known as mesoporous silica nanoparticles. This nanocarrier contains abundant packaging space in the hollow interior to incorporate cancer drugs, with the ability to carry and deliver these drugs to the walled-off and difficult to reach pancreas cancer site.
In the current carrier version, we show the loading of the chemo drug, Irinotecan, which could be delivered to the cancer site with better pharmacokinetics than the free drug. The carrier was further adapted by coating the particle surface with a lipid bilayer, which resembles a cell membrane, and serves to augment Irinotecan drug loading by trapping inside the particle to prevent premature drug leakage. Safety is improved as a result.
The Irinotecan particles alone are much more effective for shrinking pancreas cancer tumors in a mouse model than the free drug. Over time, we also recognized, as demonstrated in this publication, that we can use the lipid bilayer to add a second drug that complements the action of Irinotecan.
The opportunity for using a dual-drug design came into play with the discovery that the process of cancer cell killing by Irinotecan renders the cancer cells immunogenic, i.e., making them indigestible by antigen-presenting cells in the immune system. These cells have the function of presenting cancer antigens to cancer-killing lymphocytes.

© mentalmind/Shutterstock.com
Pancreatic cancer is known to be one of the most aggressive cancers, with low survival rates following diagnosis. Why is it so difficult to design treatments that overcome resistance, and how can nanotechnologies help to address this issue?
Although pancreatic cancer tumors are susceptible to chemotherapeutic drugs, these agents often fail to get into the cancer site because of a dense rope-like stroma walling the cancer off. The stroma also includes enzymes that metabolize and destroy the chemo agents.
Another major challenge is that many of the potentially strongest chemo agents (such as Irinotecan) are very toxic when injected into the bloodstream, leading to serious, even life-threatening drug side effects in patients who are often too sick to withstand the treatment. From this perspective, the development of the silicasome carrier provides a means of dramatically improving drug delivery while also providing drug safety.
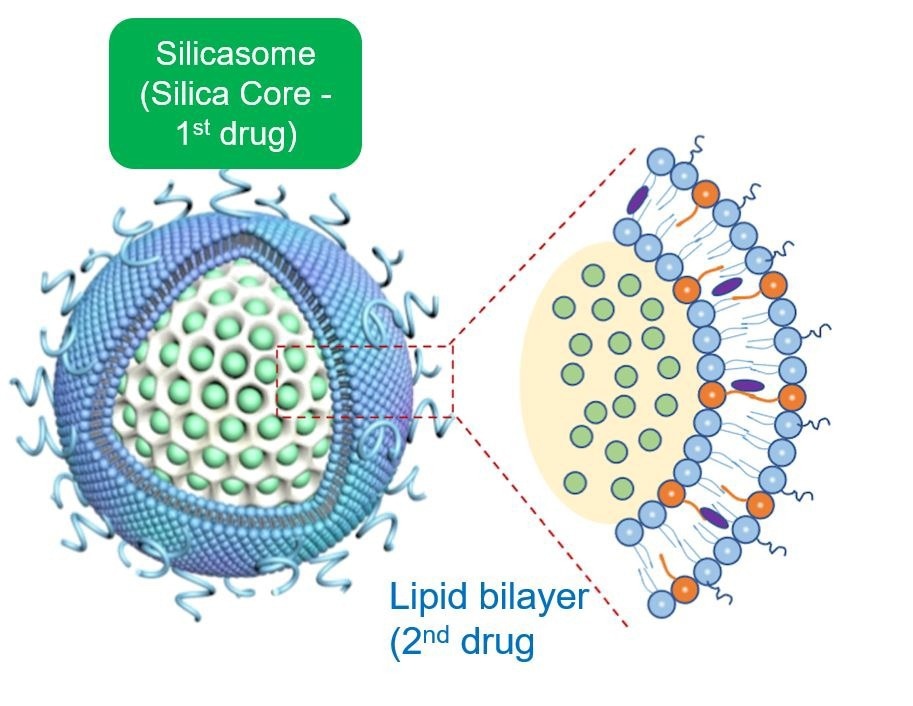
Not only is the silica core more robust than other nanocarriers, but its large packaging space allows more drug incorporation and stable retention. The resulting improvement in drug delivery and drug safety constitute two of the main principles for nanocarrier use in cancer treatment. A third major advantage of the silicasome is the ability to co-package and deliver two drugs, leading to synergy as another major advantage of using a nanocarrier.
The synergy comprises the immunogenic cell death response to Irinotecan and additional dendritic cell activation by 3M-052. Notably, a lot of the action introduced by 3M-052 takes place in surrounding lymph nodes and spleen, which assists the primary tumor site by educating cytotoxic T cells, which are then returned to the cancer site.
The nanocarrier is described as a ‘glass bubble’, and contains two drugs rather than one. How did the team approach integrating two treatments into one? What role did this ‘glass bubble’ approach have on the success of the nanocarrier’s design?
The role of the hollow silica interior (glass bubble) is to provide a large packaging space for incorporating Irinotecan for delivery to the cancer site. The unique nanoscale principle at play here is that the silica walls of the bubble serve as a surface for dense drug packaging. . Moreover, the silica backbone also provides physical strength, allowing nanoparticles to successfully migrate through the bloodstream after intravenous injection.
The silica backbone also supports the coated lipid bilayer, which serves, in turn, to seal off the drug inside the pores, preventing drug escape and toxicity. In addition, the bilayer is also used as a vehicle for loading the Irinotecan into the packaging space by a type of diffusion process known as remote loading.
Finally, the lipid bilayer incorporates additional lipid soluble drugs (such as 3M-052) to construct a dual-delivery, synergistic nanocarrier. From a nanocarrier perspective, the silicasome is representative of a variety of lipid-coated drug carriers that we have built, with the view of using the interior packaging space plus the lipid bilayer to incorporate drug combinations that strengthen and sustain anti-cancer immune responses (Nel et al., ACS Nano. 2022; 16(4): 5184-5232).
Nanocarrier Co-formulation for Delivery of a TLR7 Agonist plus an Immunogenic Cell Death 3 Stimulus Triggers Effective Pancreatic Cancer 4 Chemo-immunotherapy. © Luo et al; ACS Nano 2022; 16: 13168-13182
This ‘glass bubble’ nanocarrier approach triggers the immune system’s defense against cancer cells, rather than directly targeting the cancer cells. Why might this approach be especially effective in treating pancreatic cancers?
We have previously demonstrated that the silicasome is capable of passing through the pancreas cancer blood vessel walls, hitching a ride in small vesicles that empty their content in the cancer site, from where some of the particles gain entry to cancer cells (Liu et al., J Clin Invest; 2017; 127(5):2007-2018). Thus, Irinotecan release inside or in the vicinity of cancer cells triggers a traditional cell death response, which also involves a further element of cell stress based on the unique pharmacology of this chemo drug (Liu et al., Adv. Sci.2021,8, 2002147).
The cell stress response changes the characteristics of the dying cancer cells, rendering them attractive for uptake by antigen-presenting cells; entity description of the mode of action of Irinotecan as “immunogenic cell death.”
The ability of Irinotecan to induce immunogenic cell death in pancreatic cancer becomes particularly significant if the drug is delivered at above-threshold cytotoxic concentrations. While immunogenic cell death makes the dying cancer cell attractive for dendritic cell uptake, the functional inadequacy of these antigen-presenting cells in the pancreas cancer environment is addressed by co-delivery of 3M-052. The synergy plays out as increased killing of pancreatic cancer cells.
October 9th marks Nanotechnology Day, which aims to spread awareness about how nanoscience has benefitted our lives. Over the course of your academic career, how has the nanomedicine community grown, and what impact has it had on the reach of nanotechnology focussed science communications?
I was fortunate to contribute to the development of nanomaterial safety assessment and help to develop the field of nanomedicine under the umbrella of the National Nanotechnology Initiative in the US, with support from the National Science Foundation and the National Institute of Health (Nel A; Nano Lett. 2020, 20, 8, 5601–560; Nel et al., Science; 2006: 311:622-627).
We can look back with satisfaction on the amazing discovery and translation of nanotherapeutics efforts, which made it into the clinic within 15 years. In addition to nanocarrier contributions to improving therapeutics, bioavailability, pharmacokinetics, safety and obtaining access to difficult-to-reach disease sites, the field is in a rapid phase of expansion, now also drawing on unique material properties (other than delivery modalities) and structure-activity relationships to contribute to new modalities of imaging, sensing, diagnostics, theranostics, tissue repair, and perturbation of immune function.
Thus, we are poised to supplement the success of the first wave of nano-enabled cancer therapeutics with similar impactful advances in immunotherapy, vaccination (e.g., lipid nanoparticles in COVID-19), organ-specific pathology, infectious disease, regenerative medicine, skincare, anti-inflammatory and anti-aging applications.
The successful expansion of nanomedicine also necessitates the development of an interdisciplinary workforce and the engagement of clinicians and regulatory agencies. Cooperation between physicochemical and biomedical sciences is essential for nanomedicine to reach general acceptance in the healthcare industry.
Increased communications could help boost interest and investment in nanomedicines targeting both aggressive cancer and other high-mortality diseases. In your opinion, what are the main challenges that limit the advancement of these nanomedicines? How do you predict the field to change over the next 20 years?
What can we do to strengthen and promote the continued expansion of nanomedicine (Nel A; Nano Lett. 2020, 20, 8, 5601–560)? An important philosophical consideration is to improve understanding between the physicochemical and clinical sciences for a vibrant, multidisciplinary enterprise.
While the emphasis over the last two decades has been on materials design to impact biological outcomes, there is increasing awareness that successful nanomedicine translation should pay more attention to critical healthcare needs and the pathophysiology of the disease
The field would benefit from pushing beyond fundamental research, which emphasizes material properties to accomplish proof-of-principle outcomes, to also address important clinical challenges such as safety, material degradability, dosimetry, delivery efficacy, pharmacokinetics, production scalability, reproducible outcomes, cost-benefit analysis and the feasibility of obtaining FDA approval.
What are the next steps for this research? Are there any challenges or innovations you aim to address?
The next step for this research is to complete the preclinical study phase that is necessary to get the silicasome carrier to the clinic. One potential intended use would be to trigger pancreatic cancer chemoimmunotherapy, which can be combined with other treatment modalities.
The demonstration of successful co-formulated drug delivery promises an extension of the chemoimmunotherapy response by involving additional immunomodulators, other than 3M- 052.
This includes the use of other chemo agents that deliver immunogenic cell death responses, which can be combined with drugs and biologicals that prevent immune escape, provide immune checkpoint blockade, suppression of PD1 expression, inhibit immune metabolic pathways or overcome immune exclusion in the tumor microenvironment (Nel et al; ACS Nano; 2022; 16(4): 5184-5232).
About Professor Andre Nel
 Andre Nel is a Distinguished Professor of Medicine at UCLA, where he has successfully established a large federally-funded nanotechnology research program. Professor Nel is also board-certified as a physician in Medicine and the subspecialty of Allergy and Immunology.
Andre Nel is a Distinguished Professor of Medicine at UCLA, where he has successfully established a large federally-funded nanotechnology research program. Professor Nel is also board-certified as a physician in Medicine and the subspecialty of Allergy and Immunology.
The UC Center for the Environmental Implications of Nanotechnology (UC CEIN) emerged as one of the premier think tanks for the safe and sustainable implementation of nanotechnology in the US, while the team science efforts he has put together as Research Director of the California Nanosystems Institute is spearheading nanomedicine translation and commercialization on the UCLA campus.
Professor Nel is a recipient of the Harry Truman Award and received the 2013 California Governor’s Environmental Economic Leadership Award. He plays national leadership roles in science, biomedical research, nanotechnology and policy. He has served as the Chairs of a number of NIH study sections and was also included as a NSF panel member for producing a comprehensive US Government blueprint to further develop the Nanotechnology Initiative (NNI) from 2010-2020. He was a member of the US Bilateral Presidential Commission for technology cooperation with Russia, and served as a panel member on Pres. Obama’s PCAST panel for strategizing the NNI technological innovation and commercialization.
Professor Nel has represented the US State Department and the NIH in cooperative research agreements with Japan and the Chinese Academy of Sciences, in which he was recognized as Honorary Foreign Professor. In addition to groundbreaking work in nanotechnology, Professor Nel co-directed a leading EPA Particle Center studying the impact of air pollution on asthma. Professor Nel has been peer selected as one of the Best Doctors in America and received the John Salvaggio Award for outstanding service to the American Academy of Asthma Allergy and Immunology. He has been included as a Highly Cited Scientist (top 1% in the world of chemistry) by Clarivate analytics and is frequently invited to deliver a keynote or plenary talks at international forums. He is a serial inventor with numerous patents, contributing to the launching of startup companies.
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.