Mar 8 2009
This week's Nature Materials (09 March 2009) reveals how an international team of scientists led by researchers at the London Centre for Nanotechnology (LCN) at UCL have discovered a novel one dimensional ice chain structure built from pentagons that may prove to be a step toward the development of new materials which can be used to seed clouds and cause rain.
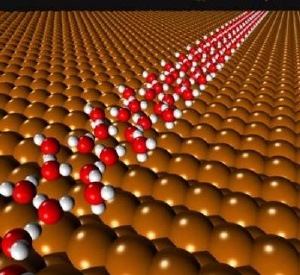 A computer generated image of a pentamer ice chain (red and white) on a plane surface (brown). Credit: LCN
A computer generated image of a pentamer ice chain (red and white) on a plane surface (brown). Credit: LCN
Although the structure of regular ice is well known at the macroscale, its structures are much more mysterious and less well understood at the nanoscale - particularly when ice forms at an interface with matter as is the case in the higher atmosphere on particles of dust. "For the first time, we have shown that ice can build an extended one dimensional chain structure entirely from pentagons and not hexagons" says Dr Michaelides. "This discovery leads to fundamental new understanding about the nature of hydrogen bonding at interfaces (there is no a priori rule that hexagons should form) and suggests that when people are searching for new ice nucleating agents which can be used to seed clouds and cause rain, they do not necessarily need to focus on materials that have hexagonal surfaces - other types of surfaces may be good too."
Ice structures are usually built out of simple hexagonal arrangements of water molecules and this hexagonal building block motif is easily observed in the structures of snowflakes. However, during their studies Dr Angelos Michaelides and co-workers from the Fritz Haber Institute, Berlin, and the University of Liverpool have discovered a natural nanoscale ice structure formed of pentagons.
"It is important to understand the structure of ice on the nanoscale, and in particular up against solid surfaces because this is how ice crystals form," explains the paper's first author Dr Javier Carrasco. "We need to understand the structure of ice crystals in the upper atmosphere because they play an important role in the formation of clouds and precipitation."
The formation of nanoscale ice crystals (i.e. nucleation) plays a key role in fields as diverse as atmospheric chemistry and biology. Ice nucleation on metal surfaces affords an opportunity to watch this process unfold at the molecular-scale on a well defined, plane interface. A common feature of structural models for such films of ice is that they are built from hexagonal arrangements of molecules.
In order to address the challenge of characterising ice on the nanoscale, the team from the LCN joined up with a team of experimentalists from the University of Liverpool (lead by Professor Andrew Hodgson) to examine ice formation on a very well defined, atomically flat copper surface. The Liverpool group performed scanning tunneling microscopy experiments and the LCN and Berlin teams carried out ab initio calculations to predict what the microscopy results would be. Only through the combination of these two state-of-the-art approaches were they able to definitively show that the ice structures that form are made from pentagons.