Jun 4 2009
A new method now enables ETH-Zurich researchers to study hundreds of cell-surface proteins simultaneously. The results obtained could help to develop more accurate diagnostic tests and more specific therapies in the future.
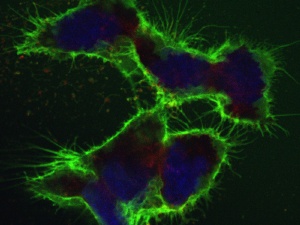 The CSC method enables hundreds of cell-surface proteins to be analyzed simultaneously. In order to produce this image, the proteins at the cell surface were labeled with green dye to make them visible; the cell nuclei are stained blue. (Photo: Wollscheid group/ETH Zurich)
The CSC method enables hundreds of cell-surface proteins to be analyzed simultaneously. In order to produce this image, the proteins at the cell surface were labeled with green dye to make them visible; the cell nuclei are stained blue. (Photo: Wollscheid group/ETH Zurich)
The CSC method enables hundreds of cell-surface proteins to be analyzed simultaneously. In order to produce this image, the proteins at the cell surface were labeled with green dye to make them visible; the cell nuclei are stained blue. (Photo: Wollscheid group/ETH Zurich) (more pictures) Cancer is a complex disease. Not all stages or subtypes of various cancers spread in the body at the same rate or respond to the same medication. For example, if a woman is diagnosed with breast cancer for instance, determining precisely which tumor grade and stage it is can be crucial in fighting the disease effectively. In many cases, however, modern medicine is not yet advanced enough to be able to do this. Standard treatments, to which not all stages and cancer forms respond, are rather the rule than the exception.
Wanted: new antibodies
In order to establish a diagnosis, usually tissue samples are studied using specific antibodies that can bind to particular proteins on the cell surface of cancer cells. To diagnose cancer, proteins or protein combinations which are typical to a particular form of cancer must be detected. The antibodies are made visible in tissue samples by coupling fluorescent dyes to them. However, as there are very few existing antibodies that work, the diagnosis largely hinges upon very few traceable proteins. The final diagnosis, then, is often simply “cancer”, the particular form is not yet detectable. In order to distinguish between different cancer stages more effectively, more accurate information regarding the assembly of the proteins on the cell surface in various cancer forms would be helpful – as would being able to detect additional, functioning antibodies for testing and diagnosing purposes.
The team of researchers headed by Bernd Wollscheid, group leader at the Institute for Molecular Systems Biology at ETH Zurich and Julian Watts from the Institute of Systems Biology in Seattle (USA), have now developed a new technology that facilitates the development of new and better antibodies. It is called the “Cell Surface-Capturing” (CSC) and permits the proteins on the cell surface to be measured more specifically. “With the CSC method we can simultaneously identify a vast number of proteins located on the surface of cells at a particular point in time”, specifies Wollscheid. And not only that: The CSC also provides information in which amounts these proteins are found – without needing any antibodies whatsoever.
Sugars on cell surface proteins are the key
The overview of the cell surface and the proteins present there is possible due to a couple of tricks: “In order to examine the cell surface proteins more specifically, we make use of the fact that they are nearly all glycoproteins”, says Wollscheid. These proteins contain at least one sugar molecule somewhere. The researchers attach a kind of adapter to these sugar molecules, which binds firmly to the sugar residue. In the next step, all the glycoproteins throughout the cell are broken into small fragments using an enzyme, which acts as a pair of molecular scissors. The researchers can then use the matching counterpart to the adaptor to easily pull out any fragments of protein that are attached to the sugar residue, i.e. which come from the cell surface. The researchers thus obtain fragments of the tagged cell surface proteins. Before they can be identified, the sugar residue and the adaptor have to be removed using another enzyme.
Developing more accurate medical diagnosis workflows
Where previously four to five proteins and their corresponding antibodies were available to characterize a somatic cell or cancer form, but now cells can now be defined via the entire assembly of their cell surface proteins. In order to distinguish between different cell types or cancer forms or stages, one can now look specifically for differences in the quantity and type of the proteins on the cell surface. This allows in the future the selection of cancer specific cell surface proteins for clinical diagnostic and the development of specific sets of antibodies.
The protein fragments obtained using the CSC technology have another crucial advantage: the fact that they origin from the exterior of the protein means that they are easily accessible for antibodies, thus making them ideal target structures for antibodies – an idea that has recently been patented. As a result, they are currently being employed in the production of antibodies.
Developing specifically acting drugs
Knowing the surface properties of particular cancer cells makes it easier to develop specific new medication that only targets this one cell type. “One could think of drugs that only bind to particular cancer cells due to their cell surface proteins, for instance, and rendering the cancer harmless”, explains Wollscheid. However, the utmost care needs to be taken in this process: if the medication is not specific enough and destroys all the kidney cells along with the breast cancer cells, the therapy will have done more harm than good.
Monitoring cell development
If the CSC method catches on, not only medicine will benefit from it but basic research will benefit as well. By taking several “snapshots” of the cell surface at particular intervals, researchers can observe how the protein composition on the cell surface changes over time. Wollscheid’s team of systems biologists illustrated this using the example of stem cells, which differentiated and developed into brain cells. “The composition of the surface proteins changed drastically”, points Wollscheid out, summing up the results. The CSC method enables the comparison of healthy and diseased cells, stem cells and differentiated ones, and to monitor the development form one form to another.
It is quite possible that the newly gained insights coupled with new diagnostic possibilities will help to develop tailored therapies for cancer patients in the foreseeable future. Nonetheless, before this goal can be achieved, much research and development work still needs to be carried out.