Jun 22 2009
A University of Pennsylvania-collaboration of bioengineers studying the physical forces generated by individual cells has created a tiny micron-sized device that allows researchers to measure and manipulate cellular forces as assemblies of living cells reorganize themselves into tissues.
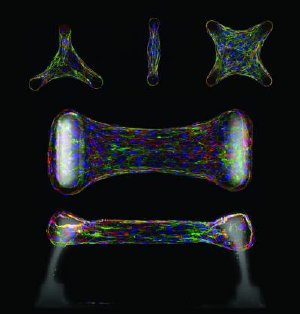 Immunofluorescence sections of cells embedded within a micropatterned collagen gel. Cells generate forces within a tissue that can feedback to regulate the accumulation of cytoskeletal proteins such as actin (green) and structural matrix proteins such as fibronectin or tenascin C (red). These forces can be measured using elastomeric cantilevers (white) that deflect in response to tissue forces. Cell nuclei are labeled in blue. Credit: Image courtesy of Wesley R. Legant
Immunofluorescence sections of cells embedded within a micropatterned collagen gel. Cells generate forces within a tissue that can feedback to regulate the accumulation of cytoskeletal proteins such as actin (green) and structural matrix proteins such as fibronectin or tenascin C (red). These forces can be measured using elastomeric cantilevers (white) that deflect in response to tissue forces. Cell nuclei are labeled in blue. Credit: Image courtesy of Wesley R. Legant
The new micro-tool created in the study allows researchers to gauge how cells' minute mechanical forces affect cellular behavior, protein deposition and cell differentiation in a 3-dimensional, in vivo-like environment that mimics how tissue actually forms in a living organism. The finding also has implications for the testing of irregular or diseased tissue, such as beating cardiac tissue, which can be modeled and studied.
The findings were published in the June issue of the Proceedings of the National Academy of Sciences.
The push-and-pull of cellular forces drives the buckling, extension and contraction of cells that occur during tissue development. These processes that ultimately shape the architecture of tissues play an important role in coordinating cell signaling, gene expression and behavior, and they are essential for wound healing and tissue homeostasis in adult organisms.
Yet a detailed picture of how tissue mechanics link to morphogenetic phenomena has been hindered by a lack of model systems in which both mechanics and remodeling can be simultaneously examined.
The Penn study highlights a complex and dynamic relationship between cellular forces, visualizes the remodeling of a matrix by living cells and demonstrates a system to study and apply this relationship within engineered 3-D microtissue.
Chris Chen, professor of bioengineering in the School of Engineering and Applied Science at Penn, developed the tool with colleagues at the University of California, Santa Barbara, and the University of Cambridge.
The system was created using photolithography, the same technology used to craft semiconductors. Scientists fabricated an array of tiny divots within a mold and immersed the mold in a culture of cells and collagen. Researchers then placed raised microcantilever posts on either side of the mold and — much like draping a volleyball net across two metal poles -- observed the formation of a cell and collagen web of living tissue anchored to the cantilevers. These microcantilevers were used to simultaneously constrain the remodeling of a collagen gel and to report forces generated during this process.
The cantilever posts allowed the team to observe and measure the retraction and extension of the cells as they remodeled the adjacent matrix into a coherent band of tissue. Varying the mechanical stiffness of the cantilevers and collagen matrix demonstrated that the cellular forces increased with boundary or matrix rigidity, whereas the levels of proteins in the cytoskeleton and extracellular matrix also increased with levels of mechanical stress. By mapping these relationships between cellular and matrix mechanics, cellular forces and protein expression onto a bio-chemo-mechanical model of microtissue contractility, the team demonstrated how intratissue gradients of mechanical stress can emerge from collective cellular contractility and, finally, how such gradients can be used to engineer protein composition and organization within a 3-D tissue.
"Just as we build muscle in the gym, these same mechanical forces are translated down to the cellular level and build the complex arrangement of different tissues in the body," co-author Wesley Legant said. "By varying the properties of our model system, we can study how these mechanical factors are distributed throughout a tissue and how this can, in turn, effect cellular function."
"With this system, we also see the potential for high-throughput drug testing, as researchers will be able to test new pharmaceuticals against a vast array of these small tissue samples, perhaps identifying new ways to increase the contractility of cardiac muscle, or to relax arteries to treat hypertension," said Chen, the study's lead author.
Working with colleagues, the team also created a mathematical model of the entire process that accurately predicted the experimental results.
"With this model, we can extend our findings to more complex and realistic model tissues which might be difficult to study experimentally in the lab" Legant said.