Dec 22 2009
Communication between nerve cells is vital for our bodies to function. Part of this communication happens through vesicles containing signalling molecules called neurotransmitters. The vesicle fuses with the nerve cell membrane; the neurotransmitters are released and quickly recorded by the next nerve cell. It is crucial that new vesicles constantly are produced for the nerve cell communication continuously to take place. If parts of this communication do not work, it leads to nerve pain like phantom pain following amputation.
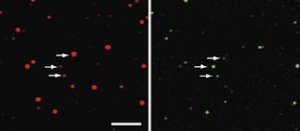 Right: Brain-lipid vesicles. Smaller "dots" indicate smaller vesicles. Left: BAR domain protein. The intensity of the dot indicates the amount of BAR bound to the vesicle. The smaller the vesicle, the more curved membrane, and the more binding of BAR. Credit: Dimitrios Stamou
Right: Brain-lipid vesicles. Smaller "dots" indicate smaller vesicles. Left: BAR domain protein. The intensity of the dot indicates the amount of BAR bound to the vesicle. The smaller the vesicle, the more curved membrane, and the more binding of BAR. Credit: Dimitrios Stamou
New discoveries on a nanoscale
- In patients with nerve pain, part of the pathological picture is a defect in a protein domain we call BAR. We have studied how BAR binds to small membrane vesicles of different size. We expect that the new knowledge can be used to combat nerve pain in the future, explains Associate Professor Dimitrios Stamou, Bio-Nanotechnology Laboratory, Nano-Science Center and the Department of Neuroscience and Pharmacology at the University of Copenhagen. Dimitrios Stamou has led the work.
- We have used nanotechnology techniques, which give us the unique opportunity to study the binding of proteins to individual vesicles. Earlier studies have been performed in solutions where you measure a large number of vesicles and proteins at a time. This gives an average value of binding and "masks out" a large number of important information that we can retrieve by measurements on single vesicles, says Dimitrios Stamou.
Error in communication
More and more studies - this study included, show that the curvature of the membrane is absolutely central to the binding of proteins to cell membranes - the greater the curvature, the greater the binding. This also applies to nerve cells in the brain. It therefore provides an important insight for the overall understanding of how nerve cells communicate with each other and for treating diseases where the communication has failed.
- To our great surprise we find that BAR binds to the membrane vesicles via small cracks in the vesicle membrane. We had expected that BAR bound to the small round membrane vesicles both because of its banana shaped structure, which fits with the shape of the vesicle, and by means of an attraction between "the banana's" positive surface and vesicle's negative surface. But instead, it is the hydrophobic part of BAR that is involved in binding, explains Dimitrios Stamou.