Berkeley Lab scientists delivered nearly 100 presentations at the American Chemical Society’s Fall 2010 national meeting in Boston, August 22-26, 2010.
In the opening scene of the famous 1967 movie The Graduate, Benjamin Braddock, at a party to celebrate his new degree, is given one word of advice for his future: “Plastics.” Were young Benjamin to be receiving that advice today the word might well have been: “Batteries.”
Economic forecasters say that the market for advanced batteries that can power electric, hybrid-electric and the emerging plug-in hybrid electric vehicles is going to be worth billions of dollars. Based on their performances in electronics and power tools, lithium-ion batteries have the potential to be far superior to nickel-metal hydride batteries but several technological issues must be addressed before they’re applied to vehicles.
Marca Doeff, a chemist with Berkeley Lab’s Materials Sciences Division, presented a talk titled “Advanced Li-ion battery cathode materials for vehicle technologies.” She focused on the cathode as one of the most expensive components in lithium-ion batteries.
“Also,” Doeff said, “the cathode is the determinant of energy density in the cell because the capacity is typically much lower than that of the graphite anode, with which it must be matched.”
Doeff and her colleagues are experimenting with various approaches for lowering the cost and improving the performance of lithium-ion cathodes. Their studies include partial substitution of the expensive cobalt constituent with aluminum, titanium or iron in layered mixed transition metal oxides now used in batteries.
So far they have found that a five-percent substitution of cobalt with aluminum increases cathode performance and cycle stability. Substitution with small amounts of titanium also led to the formation of a high-capacity and high-rate positive electrode material, whereas substitution with iron led to lower cathode capacities and poor rate capabilities.
“Our work shows that changes in electrochemical performance of the cathode depend highly on the nature of the substituting atom and its effect on the crystal structure,” Doeff said.
High Efficiency Solar Cells and other Nano Delights
At that same graduation party today, young Ben Braddock might also have been told to think “Nano.” Economic forecasters foresee an even more bountiful future for nanoscale materials, particularly in solar energy and the electronics fields. “Nanoscale electronic materials: Challenges and opportunities,” was the title of a talk by Ali Javey, a faculty scientist in Berkeley Lab’s Materials Sciences Division. In his talk Javey described a technique for engineering arrays of nanoscale pillars for a broad range of applications, including low cost, highly efficient solar cells, and artificial skin that provides prosthetic limbs with the sense of touch.
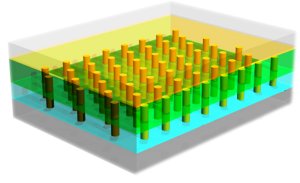
“Our technique provides large-scale assembly of highly ordered and regular arrays of nanowire components on flexible substrates through a simple contact printing process,” Javey said. “The ability to interface nanowire sensors with integrated electronics on large scales and with high uniformity presents an important advance toward the integration of nanomaterials for sensor applications.”
This technology is being applied to portable electronic and wearable human interface applications, including artificial skin. The idea is that with the integration of advanced prosthetics into the brain for better control of joints, the addition of electronic skin with nanowire sensors could enable patients to regain their sense of touch. The skin might also be used in robotics, governing how much pressure a robot applies to an object.
“Our mechanically flexible, artificial skin sensor provides impressive mechanical robustness and electrical properties,” Javey said.
Additionally, by utilizing optically active nanowire sensors, Javey and his colleagues have been able to produce highly regular, single-crystalline nanopillar arrays of semiconductors on aluminum substrates that were then configured as solar cell modules.
“Through experiments and modeling, we’ve demonstrated the that we can configure these solar modules on both rigid and flexible substrates with enhanced carrier collection efficiency and broadband photo absorption arising from the geometric configuration of the nanopillars,” Javey said. “This is a hugely promising to lower the cost of efficient solar cells.”
Hydrogen from Sunlight Via Qdot-Seeded Nanorods
One word of advice that the graduate might not have been given at the party is “hydrogen.” While experts agree that hydrogen could command a key role in future renewable energy technologies, a relatively cheap, efficient and carbon-neutral means of producing it must first be developed. The photocatalytic production of hydrogen from water using solar energy meets all the necessary criteria but there remain many materials-related obstacles to the widespread use of this approach. Berkeley Lab Director Paul Alivisatos, a chemist and leading authority on nanotechnology for energy discussed one idea for overcoming some of these obstacles in his talk titled “Photocatalytic hydrogen production with tunable nanorod heterostructures.”
In this talk, Alivisatos described a model nanosystem in which a quantum dot seed of cadmium selenide, a hydrogen-generating catalyst, is embedded inside a platinum-tipped cadmium sulfide nanorod.
“In such structures, holes are three-dimensionally confined to the cadmium selenide, whereas the delocalized electrons are transferred to the metal tip,” Alivisatos said. “Consequently, the electrons are separated from the holes over three different components and by a tunable physical length.”
The seeded nanorod metal tip facilitates efficient long-lasting charge carrier separation and minimizes back reaction of intermediates. By tuning the nanorod heterostructure length and the seed size, Alivisatos and his group are able to significantly increase hydrogen production compared to that of unseeded rods.
“We found our a multi-component nanoheterostructures to be highly active for hydrogen production, with an apparent quantum yield of 20-percemt at 450 nanometers,” Alivisatos said. “Our systems were active under orange light illumination and demonstrated improved stability compared to seedless cadmium sulfide nanorods.”