Sep 21 2010
A nanoparticle-based catalyst developed at Rice University may give that tiger in your tank a little more roar.
A new paper in the Journal of the American Chemical Society details a process by Rice Professor Michael Wong and his colleagues that should help oil refineries make the process of manufacturing gasoline more efficient and better for the environment.
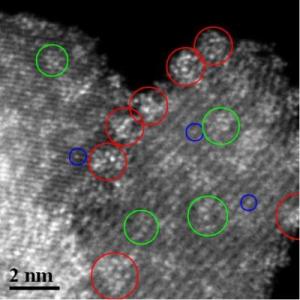 This is an atomic-level image of tungsten oxide nanoparticles (green circles) on zirconia support. The other circles show the less-active forms of tungsten oxide.
This is an atomic-level image of tungsten oxide nanoparticles (green circles) on zirconia support. The other circles show the less-active forms of tungsten oxide.
In addition, Wong said, it could produce higher-octane gasoline and save money for an industry in which a penny here and a penny there add millions to the bottom line.
Wong's team at Rice, in collaboration with labs at Lehigh University, the Centre for Research and Technology Hellas and the DCG Partnership of Texas, reported this month that sub-nanometer clusters of tungsten oxide lying on top of zirconium oxide are a highly efficient catalyst that turns straight-line molecules of n-pentane, one of many hydrocarbons in gasoline, into better-burning branched n-pentane.
While the catalytic capabilities of tungsten oxide have long been known, it takes nanotechnology to maximize their potential, said Wong, a Rice professor of chemical and biomolecular engineering and of chemistry.
After the initial separation of crude oil into its basic components -- including gasoline, kerosene, heating oil, lubricants and other products -- refineries "crack" (by heating) heavier byproducts into molecules with fewer carbon atoms that can also be made into gasoline. Catalysis, a chemical process, further refines these hydrocarbons.
That's where Wong's discovery comes in. Refineries strive to make better catalysts, he said, although "compared with the academic world, industry hasn't done much in terms of new synthesis techniques, new microscopy, new biology, even new physics. But these are things we understand in the context of nanotechnology.
"We have a way to make a better catalyst that will improve the fuels they make right now. At the same time, a lot of existing chemical processes are wasteful in terms of solvents, precursors and energy. Improving a catalyst can also make the chemical process more environmentally friendly. Knock those things out, and they gain efficiencies and save money."
Wong and his team have worked for several years to find the proper mix of active tungsten oxide nanoparticles and inert zirconia. The key is to disperse nanoparticles on the zirconia support structure at the right surface coverage. "It's the Goldilocks theory – not too much, not too little, but just right," he said. "We want to maximize the amount of these nanoparticles on the support without letting them touch.
"If we hit that sweet spot, we can see an increase of about five times in the efficiency of the catalyst. But this was very difficult to do."
No wonder. The team had to find the right chemistry, at the right high temperature, to attach particles a billionth of a meter wide to grains of zirconium oxide powder. With the right mix, the particles react with straight n-pentane molecules, rearranging their five carbon and 12 hydrogen atoms in a process called isomerization.
Now that the catalyst formula is known, making the catalyst should be straightforward for industry. "Because we're not developing a whole new process – just a component of it – refineries should be able to plug this into their systems without much disruption," Wong said.
Maximizing gasoline is important as the world develops new sources of energy, he said. "There's a lot of talk about biofuels as a significant contributor in the future, but we need a bridge to get there. Our discovery could help by stretching current fuel-production capabilities."
Source: http://media.rice.edu/