Researchers at the University of Illinois have discovered that graphene quality is based on the crystal structure of the copper substrate on which it grows based on information obtained from various imaging techniques.
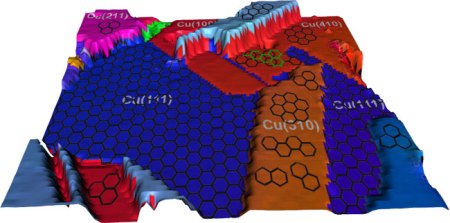 An illustration of rendered experimental data showing the polycrystalline copper surface and the differing graphene coverages. Graphene grows in a single layer on the (111) copper surface and in islands and multilayers elsewhere. Credit: Joshua D. Wood, University of Illinois
An illustration of rendered experimental data showing the polycrystalline copper surface and the differing graphene coverages. Graphene grows in a single layer on the (111) copper surface and in islands and multilayers elsewhere. Credit: Joshua D. Wood, University of Illinois
The new findings pave the way for the enhancement of high-quality graphene production. For the production of large graphene sheets, methane gas is made to flow into a furnace having a copper foil sheet. During the exposure of methane to copper, its carbon-hydrogen bonds break, resulting in the deposition of carbon onto the surface of copper and the release of hydrogen gas. The carbon atoms join together to produce graphene.
Copper is a less expensive metal and supports the growth of single-layer graphene, which is a key material for electronics applications. Although graphene developed on copper substrate is superior to the one developed on other substrates, it has multi-layer portions and flaws, which make the material unsuitable for high-performance applications. Scientists have predicted that the roughness of the copper surface has an impact on the growth of graphene. However, the University of Illinois researchers discovered that the crystal structure of the copper substrate plays a major role.
When methane is made to flow over the Copper foils, which are a cluster of various crystal structures, the shapes of the crystals encountered by methane affect the growth of graphene. The researchers allocated index numbers for different crystal shapes. Utilizing various sophisticated imaging techniques, they observed that copper patches with higher index numbers have the tendency to grow low-quality graphene. They also discovered that two familiar crystal structures, indexed 100 and 111, have demonstrated the worst and optimal growth respectively. The crystals indexed as 100 have a cubic shape and their atoms have broad gaps between them, while the crystals indexed as 111 have a tightly packed hexagonal structure.
The University of Illinois research team predicts that copper foil production with more quantity of (111) crystals may be possible. Graphene developed on these foils will not be perfect, but can be used for many applications. Further, the scientists plan to utilize their technique to analyze the growth of other two-dimensional substances such as insulators to enhance the performance of the graphene device. They also intend to record their findings by developing graphene on single-crystal copper.