Rice University researchers have discovered a novel technique to make graphene suitable for use in organic chemistry. They found a way to attach organic molecules to graphene.
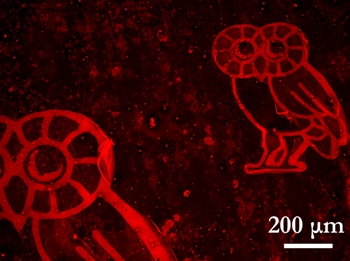 Researchers at Rice printed Owls, the university's mascot, in hydrogen atoms on a graphene substrate, turning it into a graphane superlattice suitable for organic chemistry. As proof, they "lit up" the Owls by coating them with a fluorophore and viewing them through fluorescence quenching microscopy. Graphene quenches fluorescence, but the molecules shine brightly when attached to the superlattice. Credit: Zhengzong Sun/Rice University
Researchers at Rice printed Owls, the university's mascot, in hydrogen atoms on a graphene substrate, turning it into a graphane superlattice suitable for organic chemistry. As proof, they "lit up" the Owls by coating them with a fluorophore and viewing them through fluorescence quenching microscopy. Graphene quenches fluorescence, but the molecules shine brightly when attached to the superlattice. Credit: Zhengzong Sun/Rice University
The two-step method was developed at chemist James Tour’s lab at Rice. The novel method converted a sheet of carbon having the thickness of a single atom into a superlattice.
The carbon-carbon bonds in graphene have a robust nature, which contribute to its strength. Graphene, by itself, is inert to lots of organic reactions. And as it is a semimetal it is does not have any band gap. These factors limit its applications in electronics.
Rice's W.F. Chao Chair in Chemistry, T.T. and professor of mechanical engineering and materials science and of computer science, Tour, stated that so far there were no methods to attach molecules to the plane of a graphene sheet, as they tended to go the edges, but not to the center. The two-step method can be used to hydrogenate graphene so as to make a specific pattern and then attach molecules to the places where the hydrogens were.
The first step involves creation of a lithographic pattern that can coax hydrogen atoms to attach to particular places on the graphene's honeycomb matrix. The restructuring converted the material into graphane, a two-dimensional, semiconducting superlattice. The researchers then dropped Rice University's Owl mascot and microscopic text onto a very small sheet and then used a fluorophore for spin coating. The mascot was just three times human hair width. Graphane does not quench fluorescent molecules like graphene. The researchers used fluorescence quenching microscopy (FQM) to view, which lit up the Owl mascot. They were able to view patterns having resolution of just 1ì.
In the second step, the material was exposed to diazonium salts, which attacked the carbon-hydrogen bonds of the graphane islands. The salts eliminated the hydrogen atoms and left behind a carbon-carbon sp3 bond structure. This structure can be functionalized with other organics.
The discoveries can help make advances in graphene-based metamaterials, thermoelectric devices and chemical sensors. The findings were published in the journal, Nature Communications.