A team of scientists from various research facilities including the Lawrence Berkeley National Laboratory (Berkeley Lab) has developed a metamolecule whose chirality can be switched optically at the speed of light.
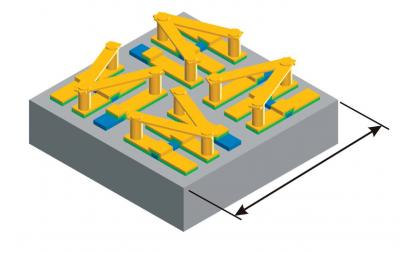 The schematic shows the chirality switching metamolecule consists of four chiral resonators with fourfold rotational symmetry. An external beam of light instantly reverses the metamolecule’s chirality from right-handed to left-handed. Credit: Courtesy of Xiang Zhang, et. al
The schematic shows the chirality switching metamolecule consists of four chiral resonators with fourfold rotational symmetry. An external beam of light instantly reverses the metamolecule’s chirality from right-handed to left-handed. Credit: Courtesy of Xiang Zhang, et. al
Chirality refers to the orientation of certain molecules toward the left or right side. Such directional forms of molecules are termed as enantiomers which have been known to demonstrate distinctly different properties. A typical illustration of this is the chiral molecule limonene that has one enantiomers smelling of oranges and the other of lemon. The new technique that enables the switching of molecular chirality by employing light of terahertz frequency could pave the way for numerous applications across a host of fields such as homeland security, biomedical studies and ultra-fast communications.
The process for switching the chirality of natural materials is slow and weak and necessitates structural changes. Previous experiments have only demonstrated switching off and on of chirality by means of photoelectric simulation. The research team engineered the chiral metamolecule by employing terahertz metamaterials developed from nanoscale gold strips with air serving as the dielectric. The molecule was integrated with a photoactive silicon medium. The team was able to observe the chirality induced by photo-excitation from an external beam of light through circularly polarized terahertz light emission. The key to the method was to combine two meta-atoms of opposite chirality into a single metamolecule. The incorporated silicon lent to the chirality of the combined molecule by breaking the mirror symmetry. The silicon also served the function of an optoelectronic switch under photo-excitation. The team envisages terahertz radiation controlled chirality to be applied in various spheres such as chemical, biological and communication sectors owing to its non-invasive property and a frequency that is closer to molecular vibration frequency range.