A research team comprising Chris Ackerson from the Colorado State University and Hannu Häkkinen from the University of Jyväskylä’s Nanoscience Center, has performed the first structural atomistic analysis of a ligand-exchange reaction process of Au102(p-MBA)44, a well-defined gold nanoparticle comprising 102 gold atoms and 44 ligand sites in the molecular overlayer.
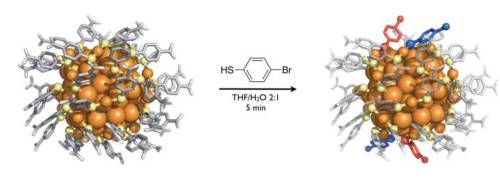 Visualization of the atomic structure of the Au102(p-MBA)44 particle (left) and the partially ligand-exchanged Au102(p-MBA)40(p-BBT)4 (right). The exchanged ligand bromo benzene thiol (p-BBT) is schematically shown in the middle and the observed ligand exchange sites by red and blue on the right.
Visualization of the atomic structure of the Au102(p-MBA)44 particle (left) and the partially ligand-exchanged Au102(p-MBA)40(p-BBT)4 (right). The exchanged ligand bromo benzene thiol (p-BBT) is schematically shown in the middle and the observed ligand exchange sites by red and blue on the right.
A water-soluble thiol (para – mercapto benzoic acid, p-MBA) was utilized as the stabilizing molecule in the synthesis of Au102(p-MBA)44. It is essential to modify this protecting molecular overlayer in virtually all applications of gold nanoparticles. The study results have been reported in the Journal of the American Chemical Society.
In this study, Ackerson’s team successfully developed heterogeneous crystals of Au102 sample particles, wherein a ligand-exchange reaction took place to partially convert the p-MBA thiols in the molecular overlayer into para – bromo benzene thiol (p-BBT) within a 5-minute reaction.
The study of the heterogeneous crystals demonstrated which ligand sites in the molecular overlayer are likely to be modified during the reaction time. The results showed that only 4 of the 44 sites had been occupied by the exchanged ligand. Theoretical study conducted by Häkkinen’s team provided insight into the atomistic description of potential reaction mechanisms.
The experimental and theoretical analysis proved that the Au102(p-MBA)44 nanoparticle comprises a thiol overlayer in which virtually each thiol ligand site demonstrates its own reaction rate because of the overlayer’s highly heterogeneous structure.
Ackerson explained that the Au102(p-MBA)44 nanoparticle’s structure resembles that of a protein, featuring a rigid inorganic gold core similar to the protein’s alpha-carbon backbone and chemically changeable functional groups in the low-symmetry molecular overlayer.
Häkkinen informed that better understanding of ligand exchange reactions is helpful to completely manipulate the surface functionalization of the Au102 and other water-soluble gold nanoparticles. The implications for a completely controllable synthetic surface of protein size are insightful.