In a nano-scale study on battery materials which appeared in Nano Letters, a research team headed by Chongmin Wang from the Pacific Northwest National Laboratory (PNNL) of the Department of Energy (DOE), has described the mechanism whereby nickel creates a physical barrier that hinders lithium ion transfer in the electrode, thus decreasing the charging and discharging rates of materials.
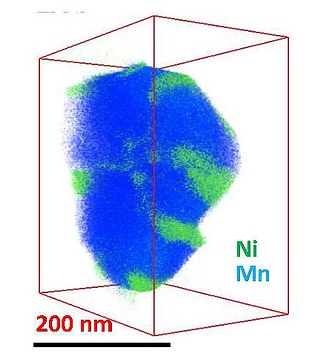
While manganese (blue) fills out this battery nanoparticle evenly, nickel (green) clumps in certain regions, interfering with the material's smooth operation. (credit: Chongmin Wang/PNNL)
To find the reason behind the decline of the capacity of a battery after repeated usage, the research team utilized electron microscopy at the Environmental Molecular Sciences Laboratory, a DOE user facility on the campus of PNNL, and the Lawrence Berkeley National Laboratory’s National Center for Electron Microscopy to observe the arrangement of different atoms in the electrode materials created by researchers at Argonne National Laboratory.
Using three-dimensional composition mapping technique, the team captured high-resolution three-dimensional images of the electrode materials made of lithium-nickel-manganese oxide layered nanoparticles to map their individual elements. These maps demonstrated the formation of clumps by nickel at some locations in the nanoparticles. Internally, the nickel accumulated on the rims of tinier regions dubbed grains. A higher magnification analysis revealed that the nickel blocked the channels whereby the lithium ion transfer normally occurs during the charging and discharging of batteries. The movement of the nickel was better than that of the lithium in the channels, thus resulting in the block.
The researchers utilized various techniques to produce the nanoparticles. Wang informed that the team wants to a find a solution to eliminate the block which forms during the production process. During fabrication, higher the duration of the exposure of the nanoparticles to high temperature, more the nickel isolated and lower the performance of the particles in charging and discharging tests.
The research team plans to perform more tightly controlled tests to identify a specific production technique to fabricate better electrodes.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.