Oct 25 2012
Mucus coats our airways' internal surfaces. The viscous gel humidifies the lungs and prevents viruses and other small particles like diesel soot from entering the body unchecked. Previously unclear was the extent to which such nanoparticles are able to move through the lungs' mucus. Here, the research evidence was contradictory.
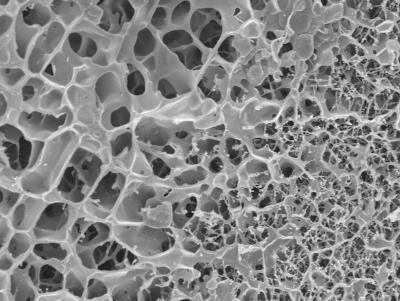 "Scientists at the Saarland University and the Helmholtz Centre for Infection Research unraveled lung mucus’s physical properties: They discovered that a rigid gel scaffold in lung mucus separates large, fluid-filled pores and prevents nanoparticle movement beyond individual pore boundaries."
"Scientists at the Saarland University and the Helmholtz Centre for Infection Research unraveled lung mucus’s physical properties: They discovered that a rigid gel scaffold in lung mucus separates large, fluid-filled pores and prevents nanoparticle movement beyond individual pore boundaries."
Scientists could not explain why, in inhaled medication development, drug nanoparticles often simply got stuck in the mucus never making it to their target destination inside the lung cells.
Now, as part of a German Research Foundation (DFG)-funded study, pharmacists and physicists were finally able to shed light on this dilemma. Scientists from the Helmholtz Institute for Pharmaceutical Research Saarland (HIPS), a branch of the HZI, together with researchers from the Saarland University, the Université Paris-Diderot, and Fresenius Medical Care Germany collaborated on the study. "The mucus inside the lungs is a very special kind of gel. Its structure is very different from other gels," explains Claus-Michael Lehr, Professor for Biopharmacy and Pharmaceutical Technology at the Saarland University and head of the "Drug Delivery" Department at HIPS. "Normal" gels have a microstructure that resembles a delicate spiderweb made from thin, very fine threads that enclose small pores. When viewed under the microscope, lung mucus, by comparison, looks more like a sponge, with rigid, thick gel rods separating large pores filled with liquid gel. "These scaffold proteins are called mucins," explains Professor Lehr. The researchers have now shown that nanoparticles become stuck at these structures as though they were bars of a cage. The explanation for why many investigations found nanoparticles in the mucus to be highly mobile is because the research was done on a nanometer scale. Inside the pores, the particles can move around completely unobstructed and only when they try to move past individual pores are they prevented from doing so by the "bars."
"Our results are helping us to better understand the etiology of infectious diseases of the airways and how to treat them more effectively. In particular, they represent an important basis for the continued development of new inhaled medications," explains Professor Lehr. The newly gained insights show that it is important to consider how drugs overcome the mucus gel scaffold. Mucolytic techniques can be used where, essentially, the rods are melted such that they dissolve before the nanoparticle and, once the particle has passed, they fuse again.
One of the research tools Professor Christian Wagner and his team of experimental physicists at the Saarland University use to support their assumptions are optical tweezers: Bundled laser beams are used to grab and move the smallest particles just like you would use a regular pair of tweezers. "We can use the optical tweezers' laser beams to measure the force that is required to move a particle within the gel. This allows us to make conclusions about the medium that the bead is moved through," explains Professor Wagner. "We were able to pull the bead through the liquid inside the pore at a constant force – just as we would if we were dealing with a normal gel. However, whenever the bead hits the pore's wall, in other words the mucus's gel rods, the laser beam is unable to move it any further," explains Wagner. Experiments using an atomic force microscope as well as other tests are further supporting their hypothesis: As such, iron nanoparticles were able to penetrate the "normal" reference gel but not the lung mucus without any difficulties under the influence of a magnetic field. Structural analyses of the mucus were performed by scientists at Fresenius Medical Care Germany using a cryo-electron microscope.
The researchers expect that insights into the special structure of lung mucus will help guiding the development of a new generation of drugs to treat diseases of the airways.