Veredus Laboratories, a leading supplier of innovative molecular diagnostic tools, has launched VereMTB™, a multiplexed molecular diagnostic chip capable of fast and accurate detection of Mycobacterium Tuberculosis Complex (MTBC) and its mutations, as well as 9 other clinically relevant non-tubercular mycobacterium. These mutations are responsible for resistance to multiple drugs and are reinvigorating the global spread of Tuberculosis.
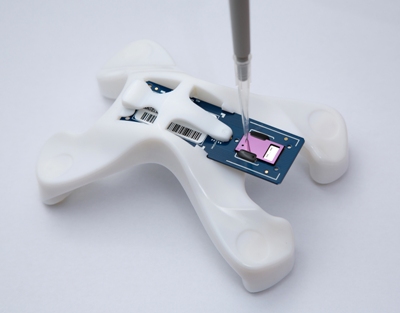 VereMTB
VereMTB
The rise of multi-drug-resistant Tuberculosis (TB) is a global healthcare challenge. Effective treatment of TB involves accurate and fast diagnosis followed by a strict regimen of the right drugs. Shortcomings in this treatment can cause the TB infection to mutate into drug-resistant strains that can become increasingly difficult and expensive to treat. Conventional methods of accurately identifying TB infections can take up to 8 weeks. In contrast, VereMTB can complete the diagnosis and identify the specific mycobacterium causing the infection and drug resistance in less than 3 hours from natural samples*, avoiding the need for culturing, the most time-consuming part of the traditional method. Additionally due to its compact size, the system can be deployed in a wide range of settings at point-of-need.
"In 2011, 8.7 million people were diagnosed with TB and 1.4 million people died from the disease. With its ranking by the World Health Organization as the second greatest killer from a single infectious agent worldwide[1], faster diagnosis and appropriate treatment of this highly infectious disease is critical," said Dr. Rosemary Tan, Chief Executive Officer of Veredus Laboratories. "We believe VereMTB fulfills a crucial need in the timely diagnosis of TB and its multi-drug resistance thus ensuring proper treatment."
The VereMTB multiplexed molecular diagnostic Lab-on-Chip was designed and tested through the TM-REST** program, as part of the European 7th Framework to develop new diagnostics to fight TB and malaria.
"STMicroelectronics is at the forefront of creating silicon technology targeted at healthcare applications," said Benedetto Vigna, Executive VP & General Manager, Analog, MEMS & Sensors Group, STMicroelectronics. "Our sensor and power-related technologies have enabled numerous innovations in healthcare, ranging from personal and portable to miniaturized and minimally-invasive devices. Using ST's Lab-on-Chip technology, VereMTB is a clear demonstration of ST's ability to leverage its technology portfolio as a strategic solution to address a global healthcare burden."
Based on STMicroelectronics' industry-proven Lab-on-Chip technology, the VereMTB chip is currently undergoing evaluations by the Chinese Center for Disease Control and Prevention in Beijing, China as part of their ongoing program to assess new technologies for TB diagnostics. According to the 2012 World Health Organization report on TB, India and China combined have almost 40 percent of the world's TB cases, and nearly 60% of multi-drug resistant cases in 2011 were in India, China, and the Russian Federation.
"At the main CDC National TB Reference Lab in Beijing, we have been evaluating VereMTB using samples, collected from across China with a special interest in detecting challenging multi-drug resistant strains that are difficult to detect using other methods," said Professor Zhao Yanlin Director of National TB Reference Laboratory and Vice Director of the National Center for Tuberculosis Control and Prevention at the Chinese Center for Disease Control and Prevention. "The speed, accuracy and comprehensiveness of the results have been very promising. We look forward to continuing our collaboration with Veredus for new breakthroughs in diagnosing TB."
VereMTB will be on display at the 43rd Union World Conference on Lung Health in Kuala Lumpur, Malaysia on 13 - 17 Nov 2012 - Booth 67, Kuala Lumpur Convention Centre. Veredus Laboratories is the Singapore-based subsidiary of STMicroelectronics (NYSE: STM), a global semiconductor leader in sensor technologies, including biosensors such as the Lab-on-Chip.
Notes to Editors:
* Natural samples refer to direct sputum coughed out by TB patients.
** The European funded consortium included STMicroelectronics, University of Sienna (Italy), University of Glasgow (United Kingdom), University of London (United Kingdom), Samara Oblast TB Service (Russian Federation), National Reference Center for Mycobacterium (Germany), University Hospital of Lung Diseases (Albania), National Centre for Infectious and Parasitic Diseases (Bulgaria), and Foundation for Innovative New Diagnostics (Switzerland). The lead clinical development member was the San Raffaele Scientific Institute of Milan, Italy. As part of the program VereMTB was tested on samples from Samara (Russian Federation), Sofia (Bulgaria), and Tirana (Albania). VereMTB has also recently undergone trials in Kampala, Uganda.