Jan 4 2013
Plasmonic gold nanoparticles make pinpoint heating on demand possible. Now Rice University researchers have found a way to selectively heat diverse nanoparticles that could advance their use in medicine and industry.
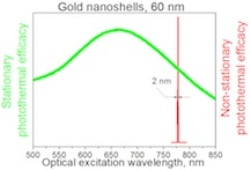 Rice University researchers found that pulsed (or “nonstationary”) lasers could narrow the response spectra of 60-nanometer-wide gold nanoshells to a very narrow spectral band (red peak), as opposed to continuous (“stationary”) excitation by laser (green peak). The discovery opens new possibilities for the use of metallic nanoparticles in medical and electronic applications. (Credit: Lapotko Group/Rice University)
Rice University researchers found that pulsed (or “nonstationary”) lasers could narrow the response spectra of 60-nanometer-wide gold nanoshells to a very narrow spectral band (red peak), as opposed to continuous (“stationary”) excitation by laser (green peak). The discovery opens new possibilities for the use of metallic nanoparticles in medical and electronic applications. (Credit: Lapotko Group/Rice University)
Rice scientists led by Dmitri Lapotko and Ekaterina Lukianova-Hleb showed common gold nanoparticles, known since the 19th century as gold colloids, heat up at near-infrared wavelengths as narrow as a few nanometers when hit by very short pulses of laser light. The surprising effect reported in Advanced Materials appears to be related to nonstationary optical excitation of plasmonic nanoparticles. Plasmons are free electrons on the surface of metals that become excited by the input of energy, typically from light. Moving plasmons can transform optical energy into heat.
“The key idea with gold nanoparticles and plasmonics in general is to convert energy,” Lapotko said. “There are two aspects to this: One is how efficiently you can convert energy, and here gold nanoparticles are world champions. Their optical absorbance is about a million times higher than any other molecules in nature.
“The second aspect is how precisely one can use laser radiation to make this photothermal conversion happen,” he said. Particles traditionally respond to wide spectra of light, and not much of it is in the valuable near-infrared region. Near-infrared light is invisible to water and, more critically for biological applications, to tissue.
“This was the problem,” Lapotko said. “All nanoparticles, beginning with solid gold colloids and moving to more sophisticated, engineered gold nanoshells, nanorods, cages and stars, have very wide spectra, typically about 100 nanometers, which means we were allowed to use only one type of nanoparticle at a time. If we tried to use different types, their spectra overlapped and we did not benefit from the high tunability of lasers.”
The discovery allows controlled laser pulses to tune the absorbance spectrum of plain gold colloids, Lapotko said. “This novel approach is counter to the established paradigm that assumes optical properties of nanoparticles are pre-set during their fabrication and stay constant during their optical excitation,” he said.
The Rice lab showed basic colloidal gold nanoparticles could be efficiently activated by a short laser pulse at 780 nanometers, with an 88-fold amplification of the photothermal effect seen with a continuous laser. The researchers repeated their experiment with nanoparticle clusters in water, in living cancer cells and in animals, with the same or better results: they showed spectral peaks two nanometers wide. Such narrow photothermal spectra had never been seen for metal nanoparticles, either singularly or in clusters.
The effect appears to depend on vapor nanobubbles that form when the particles heat liquid in their immediate environment. The nanobubbles grow and burst in an instant. “Instead of using the nanoparticle as a heat sink with a continuous, stationary laser, we’re creating a transient, nonstationary situation in which the particle interacts with the incident laser in a totally different way,” Lapotko said. He said the effect is repeatable and works with laser pulses shorter than 100 picoseconds.
Even better, an experiment with mixed nanorods and nanoshells found they responded to laser pulses with strong, distinct signals at wavelengths 10 nanometers apart. That means two or more types of nanoparticles in the same location can be selectively activated on demand.
“The nanoparticles we used were nothing fancy; they were used in the 19th century by Michael Faraday, and it was believed they could do nothing in the near-infrared,” he said. “That was the major motivation for people to invent nanorods, nanoshells and the other shapes. Here, we prove these inexpensive particles can behave quite well in near-infrared.” He said the discovery opens the possibility that many metal nanoparticles could be used in biomedical and industrial applications where spectral selectivity and tuning would provide “unprecedented” precision.
“This is still more a phenomenon rather than a firmly established mechanism, with a nice theoretical basis,” Lapotko said. “But when fully clarified, it could become a universal tool.”
Co-authors of the paper are Alexey Volkov, a research scientist at the University of Virginia, and Xiangwei Wu, an associate professor in the Department of Head and Neck Surgery at the University of Texas MD Anderson Cancer Center. Lapotko is a faculty fellow in biochemistry and cell biology, and Lukianova-Hleb is a research scientist at Rice.
The National Institutes of Health supported the research.