Like the Roman god Janus, methane presents Earth’s atmosphere with two situational faces. As the main component of natural gas, methane when burned as a fuel produces less carbon dioxide than the burning of oil or coal, which makes it a plus for global climate change.
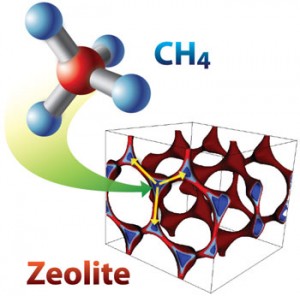 Image shows methane capture in zeolite SBN where blue represents optimal adsorption sites for methane uptake and yellow arrow shows interaction distance
Image shows methane capture in zeolite SBN where blue represents optimal adsorption sites for methane uptake and yellow arrow shows interaction distance
However, pure methane released into the atmosphere via leaks from unconventional oil and gas extraction, coal mining or from the melting of Arctic ice is an even more potent greenhouse gas than carbon dioxide, contributing an estimated 30-percent of current net climate warming. To exploit the good and blunt the bad, effective ways of separating and capturing methane must be found. This presents a huge challenge, however, as methane, unlike carbon, interacts poorly with most other materials, making it difficult to physically capture.
Berend Smit, an international authority on molecular simulations who holds joint appointments with Berkeley Lab’s Materials Sciences Division and UC Berkeley, led a computational study that found several promising candidates for methane capture in zeolites, porous minerals widely used as alkane-cracking catalysts in oil refinement. Working with a collaboration that included scientists from Lawrence Livermore National Laboratory (LLNL), Smit and his colleagues conducted systematic in silico studies on the methane capture effectiveness of two different materials systems, nanoporous zeolites and liquid solvents. None of the liquid solvents, including ionic liquids, tested as being effective, but from more than 87,000 zeolite structures, candidates were discovered that have sufficient methane sorption capacity and appropriate selectivity to be technologically promising.
“Our computational approach lets us screen hundreds of thousands of candidate structures within days, thus enabling the discovery of novel structures that can serve as the building blocks of real, practical technology,” says Smit, who directs UC Berkeley’s Energy Frontier Research Center. “These screening studies show that nanoporous materials with the right geometric constraints are able to enrich the methane concentration of low quality natural gas and coal-mine ventilation air. The next step is to see whether these in-silico studies can be used to guide the synthesis of these materials.”
The most promising of the zeolite candidates was “SBN,” which has a large number of binding sites that are formed in such a way as to maximize its interactions with methane. This results in what Smit and his colleagues characterize as an “extraordinarily high performance” for concentrating methane from a medium-concentration source to a high concentration. For treating coal-mine ventilation air, in which the methane streams are dilute, the best zeolites were those that feature one-dimensional channels with a diameter that is optimal for methane molecules. Zeolites ZON and FER were identified as prime candidates for this purpose.
Working with Smit on this project were Jihan Kim and Li-Chiang Lin, of Berkeley Lab, and Roger Aines, Amitesh Maiti and Joshuah Stolaroff of LLNL.