May 16 2013
Among its many talents, silver is an antibiotic. Titanium dioxide is known to glom on to certain heavy metals and pollutants. Yet other materials do the same for salt. In recent years, environmental engineers have sought to disinfect, depollute, and desalinate contaminated water using nanoscale particles of these active materials.
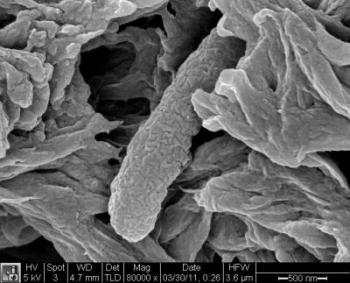 This image shows a dead E. coli bacterium (rod shape in center) collected in a filter after treatment with new Stanford nanoscavenger. Credit: Mingliang Zhang, Stanford School of Engineering.
This image shows a dead E. coli bacterium (rod shape in center) collected in a filter after treatment with new Stanford nanoscavenger. Credit: Mingliang Zhang, Stanford School of Engineering.
Engineers call them nanoscavengers. The hitch from a technical standpoint is that it is nearly impossible to reclaim the nanoscavengers once in the water.
In a paper published online May 14 in the journal Nature Communications, an interdisciplinary team of engineers at Stanford University announced it has developed a new type of nanoscavenger with a synthetic core that is ultraresponsive to magnetism, allowing the easy and efficient recovery of virtually every one of the nanoscale purifiers.
"In contaminated water, nanoscavengers float around, randomly bumping into and killing bacteria or attaching themselves to the various molecular pollutants they are after," said Shan Wang, the study's senior author and a professor of material science and engineering and jointly of electrical engineering at Stanford. "Then, when the contaminants are either stuck to the nanoscavenger or dead, the magnet is turned on and the particles vanish."
Ultraresponsive to magnetism
The use of magnetism to recover nanoscavengers is not new. There are commercial technologies today that have fashioned nanoscavengers with a core of magnetic iron oxide surrounded by an active material, but these ingenious methods are less than perfect. Iron oxide is not absolutely responsive to magnetism and too many nanoscavengers remain in the water for it to be considered safe for human use.
The Stanford advance replaces the iron oxide with a synthetic material. The Stanford core is, in reality, not a single material, but a disk of several layers. Magnetic outer layers of the synthetic material are sandwiched on either side of a titanium center, but with a twist.
"The magnetic moments of the two outer layers are opposed. That is, the direction of the magnetic force in the top layer and the bottom layer point in opposite directions, effectively canceling the magnetic properties of the material," said Mingliang Zhang, a doctoral candidate in material science and engineering and co-first author of the study.
That is to say, in their natural state, the new nanoscavengers are not magnetic. They would not be attracted to another magnetic material, for instance. When the composite discs are exposed to a strong magnetic field, however, the magnetism of the two opposing fields turn into alignment, compounding the magnetic effect.
Side-by-side tests
In doing so, the nanoscavengers become ultraresponsive to magnetism, far more so than the base iron oxide used in today's technologies. The Stanford team has dubbed their advance with the oxymoronic name: "synthetic antiferromagnetic cores." The prefix anti- in this case means in opposite direction, not non-magnetic.
With a successful core created, the researchers then cap it all with silver or titanium dioxide or other reactive material depending upon the contaminant they are targeting. In live tests using silver-capped nanoscavengers immersed in water tainted with E. coli bacteria—with a silver dosage of just 17 parts per million—the Stanford team was able to kill 99.9% of the bacteria in just 20 minutes. Better yet, they removed virtually all of the nanoscavengers in just five minutes of exposure to a permanent magnet.
Side-by-side tests of the effectiveness of the same magnet on iron-oxide-core nanoscavengers show a quick collection of about 20 percent of the nanoscavengers in the same five minutes, but then the effect plateaus. By minute 20, nearly eight-in-ten iron oxide core nanoscavengers still remain in the water.
The one-pot solution
Having demonstrated a working prototype, the team is now building various iterations of their nanoscavengers with different reactive exteriors to target specific pollutants, as well as a new class of slightly larger nanoscavengers that might bear discrete bands of several different reactants.
"Our hope is to one day create a 'one-pot solution' that tackles water afflicted by a diverse mixture of contaminants. That would be a key technology for developing and arid nations where water quality and quantity are of critical importance," added Xing Xie, a doctoral candidate in civil and environmental engineering and co-first author of the paper.