Aug 30 2013
Titanium dioxide is an inexpensive, yet versatile material. It is used as a pigment in wall paint, as a biocompatible coating in medical implants, as a catalyst in the chemical industry and as UV protection in sunscreen. When applied as a thin coating, it can keep all sorts of surfaces sparkling clean. The use of titanium oxide in the electronics industry is currently being investigated.
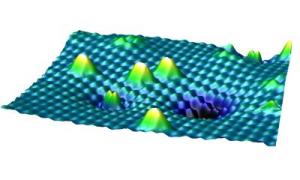 This image shows "A scanning tunneling microscope image of the surface of titanium dioxide with different forms of oxygen. The higher, white peaks are oxygen molecules that are sitting on the surface, the smaller double peak in the foreground is an oxygen molecule that is already embedded." Credit: TU Vienna
This image shows "A scanning tunneling microscope image of the surface of titanium dioxide with different forms of oxygen. The higher, white peaks are oxygen molecules that are sitting on the surface, the smaller double peak in the foreground is an oxygen molecule that is already embedded." Credit: TU Vienna
Fundamental to all these properties could be the atomic properties discovered by Ulrike Diebold from the Institute of Applied Physics at TU Vienna and Annabella Selloni from the Frick Laboratory at Princeton and their teams.
Oxygen latches on
Diebold's actual specialism is the physical and chemical properties of surfaces. "The surfaces of materials pose interesting fundamental questions, but are also important for applications", explains the physicist. The surface of titanium dioxide, for example, interacts with oxygen from the air. How this happens at the atomic level has now been shown in Vienna. Martin Setvin from Diebold's team took pictures of this surface with a scanning tunneling microscope. In this method, a fine metal tip is held extremely close to a surface, without actually touching it. A voltage is applied between the tip and the sample, which creates what is known as a tunneling current. This current is measured and displayed as an image.
Atomic vacancies pulled upwards
With this method impressive pictures are produced, in which single atoms can clearly be distinguished. By applying a high voltage between the tip and the titanium dioxide crystal, the researchers were able to pull vacancies in the atomic structure caused by single oxygen atoms that are missing to the surface and make images of them. Moreover, in a series of images, Diebold's team was able to show how differently ionised oxygen molecules become embedded in the surface.
Fuel from CO2, titanium dioxide and light?
With their results, the experimental team in Vienna were able to confirm this atomic dynamic in titanium oxide crystal, which had been previously only been predicted theoretically. "Our results clearly show how important these oxygen vacancies are for the chemical properties of titanium oxide", states Diebold about the new results from her research group. "We were also able to show that we can alter the charge state of the photocatalytically active oxygen atoms. Perhaps in future it will be possible to produce more active oxygen-rich photocatalysts. These could be used to convert CO2 into useful hydrocarbons, with the help of the titanium dioxide and light."