Sep 26 2013
A research team of Ulsan National Institute of Science and Technology (UNIST), South Korea, developed a "wormlike" hematite photoanode that can convert sunlight and water to clean hydrogen energy with a record-breaking high efficiency of 5.3%.
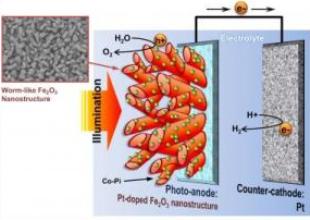 This is aSchematic Diagram of PEC cell with wormlike hematite photoanode. Credit: UNIST
This is aSchematic Diagram of PEC cell with wormlike hematite photoanode. Credit: UNIST
This research was published in Scientific Reports, a science journal published by the Nature Publishing Group. (Title: "Single-crystalline, wormlike hematite photoanodes for efficient solar water splitting") on 17 September 2013).
The previous record of solar hydrogen efficiency among stable oxide semiconductor photoanodes was 4.2% owned by the research group of Prof. Michael Graetzel at the Ecole Polytechnique de Lausanne (EPFL), Switzerland.
Solar water splitting is a renewable and sustainable energy production method because it can utilize sunlight, the most abundant energy source on earth, and water, the most abundant natural resource on earth. At the moment, low solar-to-hydrogen conversion efficiency is the most serious hurdle to overcome in the commercialization of this technology.
The key to the solar water splitting technology is the semiconductor photocatalysts that absorb sunlight and split water to hydrogen and oxygen using the absorbed solar energy. Hematite, an iron oxide (the rust of iron, Fe2O3) absorbs an ample amount of sunlight. It has also excellent stability in water, a low price, and environmentally benign characteristics.
Thus it has been a most popular and promising candidate of photoanode material for solar water splitting over the last two decades. However, hematite has a major and critical drawback of an extremely poor electrical conducting property. Thus most of the hematite anodes have exhibited very low performance.
Prof. Jae Sung Lee of UNIST led the joint research with Prof. Kazunari Domen's group at the University of Tokyo, Japan, developing new anode material which has outstanding hydrogen production efficiency.
Prof. Lee and coworkers employed a series of modifications to improve the property of hematite. First, a unique single-crystalline "wormlike" morphology was produced by using a nanomaterial synthesis technique. Second, a small amount of platinum was introduced into the hematite lattice as doping. Finally, a cobalt catalyst was employed to help oxygen evolution reaction. These modifications reduced energy loss due to charge recombination and brought the record-breaking solar-to-hydrogen conversion efficiency.
"The efficiency of 10% is needed for practical application of solar water splitting technology. There is still long way to reach that level. Yet, our work has made an important milestone by exceeding 5% level, which has been a psychological barrier in this field," said Prof. Lee. "It has also demonstrated that the carefully designed fabrication and modification strategies are effective to obtain highly efficient photocatalysts and hopefully could lead to our final goal of 10% solar-to-hydrogen efficiency in a near future."