Oct 18 2013
Graphene may command the lion’s share of attention but it is not the only material generating buzz in the electronics world.
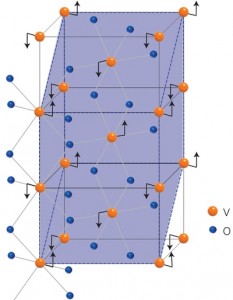 A schematic of the crystal structures in VO2, showing the motion of the vanadium (black arrows) with respect to the oxygen ions across the metal-insulator transition. VO2 acts like an insulator at low temperatures but like a metal at near room temperature.
A schematic of the crystal structures in VO2, showing the motion of the vanadium (black arrows) with respect to the oxygen ions across the metal-insulator transition. VO2 acts like an insulator at low temperatures but like a metal at near room temperature.
Vanadium dioxide is one of the few known materials that acts like an insulator at low temperatures but like a metal at warmer temperatures starting around 67 degrees Celsius. This temperature-driven metal-insulator transition, the origin of which is still intensely debated, in principle can be induced by the application of an external electric field. That could yield faster and much more energy efficient electronic devices.
“If the origin of this metal-insulator transition is electronic, the application of an electric field should trigger the transition on a picosecond or faster time-scale,” says Nagaphani Aetukuri at the IBM-Stanford Spintronic Science and Applications Center (SpinAps). “This would be the basis for an ultrafast electronic switch, in which devices would be activated so quickly that very little energy would be lost through dissipation.”
To determine the origin of the metal-insulator transition of vanadium dioxide, Aetukuri and a collaboration of researchers led by Stuart Parkin, of SpinAps and the IBM Almaden Research Center and Hermann Dürr of the SLAC National Laboratory, studied thin films of the material at Berkeley Lab’s Advanced Light Source (ALS). Using ALS beamline 4.0.2, an undulator beamline that can provide soft X-rays with variable linear polarization, they performed a series of strain-, polarization- and temperature-dependent X-ray absorption spectroscopy tests, in conjunction with X-ray diffraction and electrical transport measurements.
“Our results outlined the electronic properties that govern the metal-insulator transitions in vanadium dioxide and identified for the first time the respective roles of the Pi-symmetry and delta-symmetry electron orbitals,” Aetukuri says. “We believe that the metallic phase of vanadium dioxide can be stabilized by populating the Pi-Symmetry orbitals, which means that engineering devices on a nanoscale that can selectively transfer electrons to the Pi-symmetry orbitals should trigger an insulator to metal transition.”
This study was made possible by the X-ray beams at ALS beamline 4.0.2, which penetrated the vanadium dioxide thin films to a depth of about five nanometers, providing a bulk-sensitive probe with minimal effects from surface adsorbates.
Elke Arenholz, an ALS scientist who manages beamline 4.0.2, explains. “It was crucial for the experiment to be performed at a beamline where the orientation of the beam could be changed from parallel to perpendicular without moving the sample. Moreover, beamline 4.0.2 also provided the stability and accuracy needed to measure nanoscale effects.”
The results of this research have been reported in the journal Nature Physics in a paper titled “Control of the Metal-Insulator Transition in Vanadium Dioxide by Modifying Orbital Occupancy.” Aetukuri is the lead author, Parkin is the corresponding author.