Oil and water don’t mix, as any chemist or cook knows. Tom Russell, a polymer scientist from the University of Massachusetts who now holds a Visiting Faculty appointment with Berkeley Lab’s Materials Sciences Division, is using that chemical and culinary truth to change the natural spherical shape of liquid drops into ellipsoids, tubes and even fibrous structures similar in appearance to glass wool.
Through the combination of water, oil and nanoparticle surfactants plus an external field, Russell is able to stabilize water drops into non-equilibrium shapes that could find valuable uses as therapeutic delivery systems, biosensors, microfluidic lab-on-a-chip devices, or possibly as the basis for an all-liquid electrical battery.
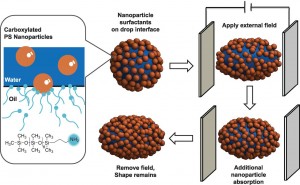 Schematic shows how the application of an electrical field transforms a spherical drop clad with nanoparticle surfactants into an ellipsoid.
Schematic shows how the application of an electrical field transforms a spherical drop clad with nanoparticle surfactants into an ellipsoid.
“Using the in situ formation of nanoparticle surfactants on a water drop that’s been suspended in oil, we’ve demonstrated a simple route to produce and stabilize fluid drops having shapes far removed from their equilibrium spherical shape,” says Russell.
In a study he carried out at UMass with Mengmeng Cui and Todd Emrick, a drop of water was suspended in silicone oil and carboxylated nanoparticles were added to the water. The nanoparticles self-assembled at the oil/water interface to form a sphere-shaped surfactant drop – like a soap bubble. Applying an electric field to the drop overcame the equilibrium energy that stabilizes its spherical shape and deformed the sphere into an ellipsoid.
Since an ellipsoid has a greater surface area than a sphere of the same volume, a great many more nanoparticles can attach themselves to it. When the electric field was removed, the nanoparticle drop tried to return to the spherical shape of its equilibrium energy. However, the swollen number of nanoparticles jammed together at the oil/water interface, essentially “gridlocking” the drop into a stable ellipsoid shape.
“You can think of it like traffic getting jammed at an exit ramp or particles of sand getting jammed in an hourglass,” Russell says. “We start out by deforming a drop shaped like a basketball into a drop shaped like a football. The jamming effect locks in the football shape. If we continue the deforming and jamming process, we can create a wide assortment of shapes that are stable even though far removed from equilibrium.”
In the original experiment, a drop of water was suspended in oil, but Russell says the experiment could just as easily have been done with a drop of oil suspended in water. He also says the shape deformation can be accomplished by mechanical stirring and that the degree of deformation is determined by the strength of the applied electrical field or how long and vigorously the liquid is stirred. While he and his colleagues never changed the volume of their drops in the original study, just the shape, Russell says the volume of the drops can be inflated with the addition of more liquids, or deflated with the removal of liquids.
“When you can control the shape of one liquid in another liquid and the shapes of the liquids are locked-in you can think about microfluidic devices-devices that are completely liquid inside the drop, or reactive liquid systems for packaging, delivery and storage,” Russell says. “You can also conceive of batteries in which ions flow through water tubes. You might even make droplets that display really high shock resistance because they’re basically a liquid surrounded by another liquid.”
Russell was the corresponding author on a paper describing this work that was published in Science. The paper was titled “Stabilizing Liquid Drops in Nonequilibrium Shapes by the Interfacial Jamming of Nanoparticles.” Cui and Emrick were the co-authors.
At Berkeley Lab, he will continue to develop these concepts with responsive nanoparticle surfactants, exploring the application of magnetic and ultrasonic fields to deform droplet shapes. He will capitalize on the resources of the Advanced Light Source, the Molecular Foundry and the National Center for Electron Microscopy, all DOE national user facilities hosted by Berkeley Lab.