Jan 10 2014
The interaction of water with the surfaces of solid materials is ubiquitous. Many remarkable physical and chemical properties of water/solid interfaces are governed by H-bonding interaction between water molecules. As a result, the accurate description of H-bonding configuration and directionality is one of the most important fundamental issues in water science.
Ideally, attacking this problem requires the access to the internal degrees of freedom of water molecules, i.e. the O-H directionality. However, resolving the internal structure of water has not been possible so far despite massive efforts in the last decades due to the light mass and small size of hydrogen.
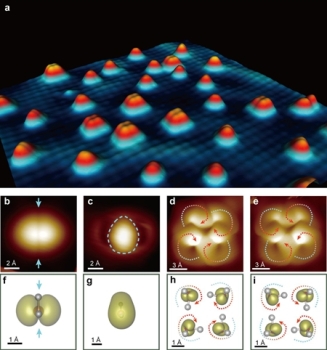 (a) 3D STM topographic image of water monomers and tetramers adsorbed on the NaCl(001) surface. (b) and (c) HOMO and LUMO images of a water monomer, respectively. (d) and (e) HOMO images of two water tetramers with different H-bonding chirality. (f)-(i) Calculated isosurfaces of HOMO and LUMO orbitals, corresponding to (b)-(e).
(a) 3D STM topographic image of water monomers and tetramers adsorbed on the NaCl(001) surface. (b) and (c) HOMO and LUMO images of a water monomer, respectively. (d) and (e) HOMO images of two water tetramers with different H-bonding chirality. (f)-(i) Calculated isosurfaces of HOMO and LUMO orbitals, corresponding to (b)-(e).
Recently, the teams led by Professor Ying Jiang and Professor Enge Wang of International Center for Quantum Materials (ICQM) of Peking University succeeded to achieve submolecular-resolution imaging of individual water monomers and tetramers adsorbed on a Au-supported NaCl(001) film at 5 K, using a cryogenic scanning tunneling microscope (STM). They first decoupled electronically the water molecule from the metal substrate by inserting an insulating NaCl layer and then employed the STM tip as a top gate to tune controllably the molecular density of states of water around the Fermi level. These key steps enabled them to image the frontier molecular orbitals which are spatially locked together with the geometric structures of water molecules. Notably, they were able to discriminate in real space the orientation of water monomers and the H-bonding directionality of water tetramers based on the submolecular-resolution orbital images.
This work opens up the possibility of determining the detailed topology of H-bonded networks at water/solid interfaces with atomic precision, which is only possible through theoretical simulations in the past. The ability to resolve the O-H directionality of water provides further opportunities for probing the dynamics of H-bonded networks at atomic scale such as H-atom transfer and bond rearrangement. In addition, the novel orbital-imaging technique developed in this work reveals new understanding of STM experiments and may be applicable to a broad range of molecular systems and materials.
This work was published online in Nature Materials on Jan. 5, 2014 [Nature Materials DOI: 10.1038/nmat3848, http://www.nature.com/nmat/journal/vaop/ncurrent/full/nmat3848.html]. This work received supports from Ministry of Science and Technology of China, National Natural Science Foundation of China, Ministry of Education of China, and Peking University.
Source: International Center for Quantum Materials
Edited by: Zhang Jiang