Mar 28 2014
With DESY's X-ray light source PETRA III, Danish scientists observed the growth of nanoparticles live. The study shows how tungsten oxide nanoparticles are forming from solution. These particles are used for example for smart windows, which become opaque at the flick of a switch, and they are also used in particular solar cells.
The team around lead author Dr. Dipankar Saha from Århus University present their observations in the scientific journal Angewandte Chemie – International Edition.
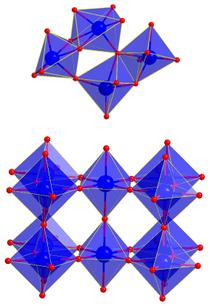 Top: Structure of the ammonium metatungstate dissolved in water on atomic length scale. The octahedra consisting of the tungsten ion in the centre and the six surrounding oxygen ions partly share corners and edges. Bottom: Structure of the nanoparticles in the ordered crystalline phase. The octahedra exclusively share corners. Credit: Dipankar Saha/Århus University
Top: Structure of the ammonium metatungstate dissolved in water on atomic length scale. The octahedra consisting of the tungsten ion in the centre and the six surrounding oxygen ions partly share corners and edges. Bottom: Structure of the nanoparticles in the ordered crystalline phase. The octahedra exclusively share corners. Credit: Dipankar Saha/Århus University
For their investigation, the scientists built a small reaction chamber, which is transparent for X-rays. "We use fine capillaries of sapphire or fused silica which are easily penetrable by X-rays," said Professor Bo Iversen, head of the research group. In these capillaries, the scientists transformed so-called ammonium metatungstate dissolved in water into nanoparticles at high temperature and high pressure. With the brilliant PETRA III X-ray light, the chemists were able to track the growth of small tungsten trioxide particles (WO3) with a typical size of about ten nanometres from the solution in real time.
"The X-ray measurements show the building blocks of the material," said co-author Dr. Ann-Christin Dippel from DESY, scientist at beamline P02.1, where the experiments were carried out. "With our method, we are able to observe the structure of the material at atomic length scale. What is special here is the possibility of following the dynamics of the growth process," Dippel points out. "The different crystal structures that form in these nanoparticles are already known. But now we can track in real-time the transformation mechanism of molecules to nanocrystals. We do not only see the sequence of the process but also why specific structures form."
On the molecular level, the basic units of many metal-oxygen compounds like oxides are octahedra, which consist of eight equal triangles. These octahedra may share corners or edges. Depending on their configuration, the resulting compounds have different characteristics. This is not only true for tungsten trioxide but is basically applicable to other materials.
The octahedra units in the solutions grow up to nanoparticles, with a ten nanometre small particle including about 25 octahedra. "We were able to determine that at first, both structure elements exist in the original material, the connection by corners and by edges," said Saha. "In the course of the reaction, the octahedra rearrange: the longer you wait, the more the edge connection disappears and the connection by corners becomes more frequent. The nanoparticles which developed in our investigations have a predominantly ordered crystal structure."
In the continuous industrial synthesis, this process occurs so quickly, that it mainly produces nanoparticles with mixed disordered structures. "Ordered structures are produced when nanoparticles get enough time to rearrange," said Saha. "We can use these observations for example to make available nanoparticles with special features. This method is also applicable to other nanoparticles."