Berkeley lab researchers find that viral packaging motor rotates DNA and adapts to changing conditions, information that could help future drug designs
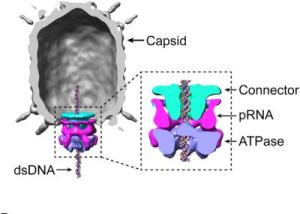 Cryoelectron microscopy reconstruction of the Phi 29 capsid (gray), connector (cyan), pRNA (magenta), and ATPase ring (blue). Credit: Image courtesy of Morais, et. Al
Cryoelectron microscopy reconstruction of the Phi 29 capsid (gray), connector (cyan), pRNA (magenta), and ATPase ring (blue). Credit: Image courtesy of Morais, et. Al
Viruses are the enigma of the biological world – despite having their own DNA and being able to adapt to their environment and evolve, they are not considered to be alive like cells. In order to reproduce and multiply – a requirement of "life" - a virus must invade a living cell, eject its DNA into that of the cell, and commandeer the cell's biological machinery. While a virus, essentially, may be nothing more than a dollop of DNA packed into a protective coating of protein called a capsid, the packaging of that DNA is critical. The molecular motors that drive this DNA packaging process, however, have remained almost as enigmatic as the viruses themselves. A better understanding of these motors could be crucial to combating viral infections.
Studying molecular motors is the signature work of Carlos Bustamante, a biophysicist who holds joint appointments with Lawrence Berkeley National Laboratory (Berkeley Lab) and the University of California (UC) Berkeley, as well as the Howard Hughes Medical Institute (HHMI) and the Kavli Energy NanoSciences Institute at Berkeley. In his latest research, he and a team of collaborators have shed new light on a type of molecular motor used to package the DNA of a number of viruses, including such human pathogens as herpes and the adenoviruses. Their findings also provide the first experimental confirmation of ideas proposed some 30 years ago.
"In a study of the DNA packaging motor of the Phi 29 virus, we have demonstrated for the first time that the motor not only exerts force on the DNA but also exerts torque to rotate it,"
Bustamante says. "We have further shown that this rotation is necessary for the motor to coordinate the activity of all its subunits during its mechano-chemical cycles. We also discovered that as the capsid fills with DNA, the motor adapts its operation to effectively throttle down and prepare itself to terminate packaging."
Bustamante is the corresponding author of a paper reporting the results of this study in the journal Cell entitled "A Viral Packaging Motor Varies Its DNA Rotation and Step Size to Preserve Subunit Coordination as the Capsid Fills." Shixin Liu, Gheorghe Chistol, and Craig Hetherington are the lead authors. Co-authors are Sara Tafoya, Aathavan Karunakaran, Joerg Schnitzbauer, Shelley Grimes and Paul Jardine.
In the 1970s, scientists proposed that the DNA within the viral capsid organizes as a spool that might require it to rotate relative to the capsid. It was also suggested that the DNA might need to rotate relative to the packaging motor in order to maintain crucial electrostatic contacts. However, until now, scientists lacked the experimental tools to prove or refute these.
Bustamante, who is a faculty scientist with Berkeley Lab's Physical Biosciences Division and UC Berkeley's Raymond and Beverley Sackler Chair of Biophysics, has been a pioneer in the study of single molecules and molecular motors using optical tweezers and microscopic beads.
In this latest effort, he and his collaborators modified their standard two-bead optical-tweezers packaging assay by introducing a third "rotor bead" that enabled them to monitor changes in the angle of the DNA around its axis while simultaneously observing DNA translocation into the viral capsid.
"We were able to follow a viral packaging motor in real-time at different stages of its biological task and discover the multiple and specific ways in which the motor's mechanisms are modified in response to external signals," Bustamante says. "We showed that by designing carefully controlled experiments, it is possible to learn a great deal about the subtle molecular mechanisms underlying the coordination of various molecular motor components."
The Phi29 virus that was the subject of this study is a bacteriophage of Bacillus subtilis, a bacterium found in soils and the human gut. Its DNA packaging motor complex consists of three coaxial rings through which DNA is threaded into the capsid. The catalytic core of the motor complex is a ring ATPase that consists of five subunits. The Phi29 packaging ATPase is considered a model for ring-shaped molecular motors that are common in living cells and rely on the coordinated action of their subunits to perform crucial biological functions.
"In a previous study we showed that the Phi 29 ring motor exhibits an interesting division of labor in that four of the five subunits are the workers that move the DNA into the capsid, and the remaining subunit is the supervisor that regulates the progression of the packaging cycles," says Liu. "However the mechanism for breaking the symmetry of the ring remained unclear. Our new results point towards a model in which the supervisory subunit is the one that maintains electrostatic contacts with the DNA backbone phosphates through every cycle by rotating the DNA. This special subunit does not normally change its identity from cycle to cycle."
The typical optical-tweezers packaging assay used by Bustamante and his group involves tethering DNA to polystyrene beads and using laser beams as optical tweezers to exert opposing forces on each bead. With the addition of the rotor bead to this assay, the researchers could not only measure pushing and pulling forces but also torque. They discovered that the packing motor has developed a surprisingly sophisticated mechanism that allows it to respond to increasing internal pressure from the encapsidated DNA.
"Previously it was generally believed that the filled DNA simply applies a resisting force that works against the power stroke of the motor to slow it down and eventually stop," Liu says. "We found that the resisting force only makes a minor contribution to the slowing down of the motor. Instead, the motor adapts its step size and amount of DNA rotation to ensure that the same subunit makes specific electrostatic contacts with the DNA backbone phosphates every cycle. Remarkably, this coordination mechanism is maintained throughout the entire packaging process even as the packaging velocity drops by two orders of magnitude."
The ability of the Phi 29 packing motor to adjust its operation to changing conditions, while maintaining its basic scheme of coordination, may be a general design principle for other ring motors as well, the researchers say.
"By elucidating the full course of viral DNA packaging, we can better understand packaging energetics and subsequent ejection, which in turn should help us develop more effective strategies to stop or block these motors and find alternative drugs against the viruses they package," Liu says. "Also, some of the characteristics found in this biological motor could inspire the design of efficient and responsive synthetic machines."