Jun 10 2014
Plant cells are beginning to look a lot different to Dr. A. Bruce Cahoon and his colleagues at Middle Tennessee State University (MTSU). They've adopted a new approach that combines the precision of an ion beam with the imaging capabilities of an electron beam to zoom in at micron-level resolution.
Scientists who work with nanodevices have used focused ion beam–scanning electron microscopy (FIB-SEM) for decades, but it is only recently that biologists have begun to explore its capabilities. The researchers at MTSU are the first to optimize its use for plant cell imaging.
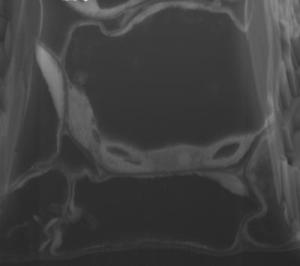 This is a high-resolution SEM micrograph from FIB-SEM manual imaging showing Arabidopsis thaliana leaf mesophyll cells with chloroplasts, nucleus, and internal membranous endoplasmic reticulum connections. Credit: Image credit Bhawana et al. From Bhawana, Joyce L. Miller, and A. Bruce Cahoon. 3D Plant cell architecture of Arabidopsis thaliana (Brassicaceae) using focused ion beam–scanning electron microscopy. Applications in Plant Sciences 2(6): 1300090. doi:10.3732/apps.1300090
This is a high-resolution SEM micrograph from FIB-SEM manual imaging showing Arabidopsis thaliana leaf mesophyll cells with chloroplasts, nucleus, and internal membranous endoplasmic reticulum connections. Credit: Image credit Bhawana et al. From Bhawana, Joyce L. Miller, and A. Bruce Cahoon. 3D Plant cell architecture of Arabidopsis thaliana (Brassicaceae) using focused ion beam–scanning electron microscopy. Applications in Plant Sciences 2(6): 1300090. doi:10.3732/apps.1300090
Their results are beautiful grayscale mosaics of plant organelles. Large, dark vacuoles bordered by light, oval-shaped chloroplasts resting along thick, smooth cell walls. Plump berry-like lipid bodies surrounding the round nucleus. Endoplasmic reticulum, protein bodies, and other uniquely shaped structures squeezing together like flexible tiles within the cells. The researchers produced a stunning selection of images as they developed methods to work with seed, leaf, stem, root, and petal cell types of Arabidopsis thaliana. Cahoon and his colleagues describe their methods and results, along with 3D renderings and video, in the June issue of Applications in Plant Sciences.
"We attended an open presentation about FIB-SEM held at MTSU about its capabilities, and we immediately thought of using it on plant tissues," explains Cahoon. The ion beam slices thin sections of a sample, which are each captured in an image by the scanning electron beam. The images of over 100 sections, just nanometers thick, are combined to construct a richly detailed three-dimensional figure.
"Certain aspects were very attractive, but it did mean we would have to step back and innovate the technology for plant tissues," says Cahoon. Fortunately, the researchers were able to benefit from the work of a few other biologists who had begun using FIB-SEM with animal tissues. The animal tissue protocol served as a base from which to modify specific plant techniques.
"The 3D visualization of subcellular structures only seen in 2D views was very satisfying," says Cahoon. "Some were just as you would imagine but others were surprisingly organized—for example, amorphous aggregates in the petal cell vacuoles that had been dismissed in previous electron microscopy studies as artifacts or uninteresting. The 3D view of these structures revealed a regular pattern appearing in almost every petal mesophyll cell used in our study. When molecules are organized like this, it suggests function and poses new questions."
Electron microscopes can produce images at a much higher magnification and resolution than optical light microscopes because electrons have shorter wavelengths than visible light. The ion beam is able to penetrate the tissue sample and slice off thin sections at a precision much greater than any form of mechanical milling. Combining these two technologies offers unique images unattainable by any other method.
Unfortunately, all good things tend to have their drawbacks and FIB-SEM is no exception. It is a time-intensive process that requires an expensive specialized instrument. Tissue samples must undergo highly specific fixation procedures to stabilize them for imaging in the electron microscope, and non-conductive biological cells require a conductive coating, such as platinum or a gold alloy.
For the research team at MTSU, the time-consuming procedures and expensive equipment are well worth the results. "We have new specific questions to address that arose from the expanded view of the internal structures. In addition, now that we know how to use this technology, we have begun to expand the repertoire of photosynthetic organisms to, at least initially, explore their cellular architecture just to see where it takes us."
The new FIB-SEM methods developed for seed, leaf, stem, root, and petal cell types will help expand the toolset available to plant anatomists for understanding the nature of organelles, cells, and plant development. A unique view of plant cell interiors could reveal never-before-seen aspects of the architecture and distribution of organelles. This work is just one example of how technological advances in one field of science, in this case, materials science, can open new doors for researchers in other fields.