Jun 28 2014
The Institute for Basic Science (IBS) has announced that the Center for Self-assembly and Complexity have succeeded in developing a new technology that introduces metal nanoparticles on the surface of polymer nanocapsules made of cucurbit[6]uril.
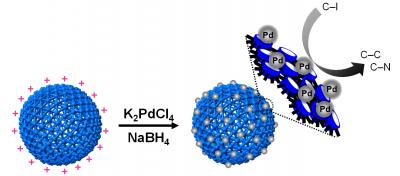 Hollow polymer nanocapsules (PNs) made of cucurbit-[6]uril (CB) serve as a versatile platform since various metal nanoparticles (NPs) can be introduced on the surface. They allow for a controlled synthesis, prevent self-aggregation, and provide high stability and dispersibility. Pd@CB-PNs show outstanding properties as heterogeneous catalysts in C-C and C-N bond-forming reactions in water. Credit: © Institute for Basic Science
Hollow polymer nanocapsules (PNs) made of cucurbit-[6]uril (CB) serve as a versatile platform since various metal nanoparticles (NPs) can be introduced on the surface. They allow for a controlled synthesis, prevent self-aggregation, and provide high stability and dispersibility. Pd@CB-PNs show outstanding properties as heterogeneous catalysts in C-C and C-N bond-forming reactions in water. Credit: © Institute for Basic Science
The researchers have found that using polymer nanocapsules made of cucurbit[6]uril and metal salts can serve as a versatile platform where equal sized metal nanoparticles can be evenly distributed on the surface of the polymer nanocapsules. Cucurbit[6]uril has properties which strongly and selectively recognize organic and inorganic chemical species. This makes it possible to use it as a protecting agent which can stabilize metal nanoparticles by preventing them from clustering together. The metal-nanoparticle-decorated polymer nanocapsules exhibit the following properties in water: high stability for up to 6 months; high dispersibility; excellent catalytic activity; and reusability in carbon-carbon and carbon-nitrogen bond-forming reactions with 100% conversion efficiency.
Even though metal nanoparticles are variously used in industrial, pharmaceutical and agricultural (fertilizer) applications as a catalyst, toxic liquids such as toluene and hexane are usually used as solvents in the carbon-carbon and carbon-nitrogen bond-forming reactions. These toxic liquid solvents raise many issues for concern including environmental pollution, high cost of disposal, health problems and poisoning during the disposal process.
However, this new technology is able to replace those toxic liquids as it allows carbon-carbon and carbon-nitrogen bond-formation with the use of metal nanoparticles as a catalyst, which has high stability in environmentally preferable solvents such as water.
"The research results demonstrated that this new technology shows high stability, dispersibility, catalytic activity, and reusability in water, which other existing metal nanoparticles on solid supports have not been able to do," says Kimoon Kim, director of the Center for Self-assembly and Complexity at IBS. "It is important as it presents new possible applications in green solvents or bioimaging and nanomedicine fields."