Jul 1 2014
As in a successful football match, all actors in a cell must play in perfect coordination. A typical example for this kind of cooperation can be seen in the heat shock protein Hsp90, which controls the proper folding of other proteins. Together with a second molecule, the co-chaperone P23, it splits the energy source ATP to release the energy it needs to do its work.
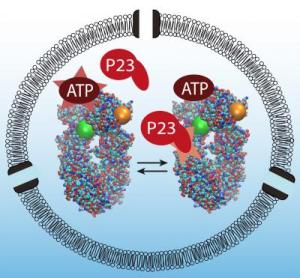 Without P23 the heat shock enzyme effectively runs on idle. When P23 joins the game, it is like shifting into gear. Credit: Bjoern Hellenkamp / TUM
Without P23 the heat shock enzyme effectively runs on idle. When P23 joins the game, it is like shifting into gear. Credit: Bjoern Hellenkamp / TUM
However, while normal enzyme reactions often are easy to follow because the involved proteins alter their conformations clearly, the interaction between P23 and ATP involves significantly less conspicuous changes in state.
Using a sophisticated methodology, a team led by Professor Thorsten Hugel, head of the Department for Molecular Machines at the TU Muenchen and member of the Excellence Cluster Nanosystemns Initiative Munich (NIM), has now managed to observe this reaction in detail for the first time – step for step in an Hsp90 molecule, P23 protein and ATP.
Live transmission of molecular processes
To this end, the team adapted the so-called FRET (Foerster resonance energy transfer) methodology to suit their requirements. The approach works by using a variety of fluorescent pigment molecules bonded to specific sites in the involved components. When these complexes are excited with light of a specific wavelength, the pigments start to fluoresce in a kind of chain reaction. The emitted fluorescent light reveals the precise distance between the marked sites, right down to the nanometer.
To determine exactly how the components Hsp90, P23 and ATP interact with each other, the biophysicists observed the positions and bonding sequences of the individual molecules over a span of several minutes. From the resulting data they could deduce even the smallest of changes, as well as the biological function of the overall complex.
Energy production as a team effort
Using this approach, the Munich researchers successfully demonstrated in detail that the P23 protein strengthens ATP bonding, thereby significantly increasing the amount of energy exploited. They also showed that the two substances bond with Hsp90 this effectively only as a team, thereby allowing ATP splitting to be used so successful.
"Without P23 the heat shock enzyme effectively runs on idle," explains Bjoern Hellenkamp their results. "When P23 joins the game, it is like shifting into gear. The energy is released and the reaction moves clearly in one direction. This is referred to as directionality."
In the near future the biophysicists want to investigate in detail how Hsp90 uses the exploited energy. The newly established methodology also allows them to investigate other multicomponent systems with mechanisms that have eluded study because of their minimal conformational alterations.
This news release is available in German.