Jul 4 2014
Deep inside the brains of people with dementia and Lou Gehrig's disease, globs of abnormal protein gum up the inner workings of brain cells – dooming them to an early death.
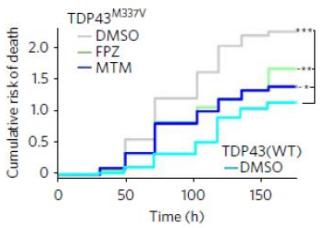 Two drugs that boost autophagy led to longer survival of neurons grown from stem cells derived from the cells of patients with ALS (middle two lines). Credit: University of Michigan/Nature Chemical Biology
Two drugs that boost autophagy led to longer survival of neurons grown from stem cells derived from the cells of patients with ALS (middle two lines). Credit: University of Michigan/Nature Chemical Biology
But boosting those cells' natural ability to clean up those clogs might hold the key to better treatment for such conditions.
That's the key finding of new research from a University of Michigan Medical School physician scientist and his colleagues in California and the United Kingdom. They reported their latest findings this week in the journal Nature Chemical Biology.
Though the team showed the effect worked in animals and human neurons from stem cells, not patients, their discoveries point the way to find new medicines that boost the protein-clearing cleanup process.
The work also shows how an innovative microscope technique can help researchers see what's going on inside brain cells, as they labor to clear out the protein buildup.
The researchers focused on a crucial cell-cleaning process called autophagy – a hot topic in basic medical research these days, as scientists discover its important role in many conditions. In autophagy, cells bundle unwanted materials up, break them down and push the waste products out.
In the newly published research, the team showed how the self-cleaning capacity of some brain cells gets overwhelmed if the cells make too much of an abnormal protein called TDP43. The found that cells vary greatly in how quickly their autophagy capacity gets swamped.
But they also showed how three drugs that boost autophagy – speeding up the clean-out process – could keep the brain cells alive longer.
Longer-living, TDP43-clearing brain cells are theoretically what people with Lou Gehrig's disease (amyotrophic lateral sclerosis or ALS) and certain forms of dementia (called frontotemporal) need. But only further research will show for sure.
Sami Barmada, M.D., Ph.D., the U-M neurologist and scientist who is first author of the new study, says the new findings are encouraging – and so is the success of a microscope technique used in the research. His new lab, in the U-M Department of Neurology, is continuing to refine ways to view the inner workings of nerve cells.
"Using this new visualization technique, we could truly see how the protein was being cleared, and therefore which compounds could enhance the pace of clearance and shorten the half-life of TDP43 inside cells," he says. "This allowed us to see that increased autophagy was directly related to improved cell survival."
Barmada worked on the team at the Gladstone Institutes and the University of California San Francisco headed by Steven Finkbeiner, M.D., Ph.D., that published the new findings. The team used stem cells derived from the cells of people who have ALS to grow neurons and astrocytes – the two types of brain cell most crucial to normal brain function.
Because he both sees patients in clinic and studies neurological disease in the laboratory, Barmada brings a special perspective to the research.
At U-M, he specializes in treating patients who have neurological diseases that affect both thinking and muscle control. About a third of ALS patients develop signs of frontotemporal dementia, also called FTD – and about 10 percent of people with FTD also have a motor neuron disease that affects their brain's ability to control muscle movement.
One of the drugs tested in the study, an antipsychotic drug developed in the 1960s to treat people with schizophrenia, had actually shown some anti-dementia promise in human ALS patients, but comes with many side effects. Barmada notes that Finkbeiner's team at the Gladstone Institute is already working to identify other compounds that could produce the effect with fewer side effects.
Interestingly, small studies have suggested that people with schizophrenia who take antipsychotic drugs are much less likely to develop ALS.
Barmada's work at U-M now focuses on the connection between brain cells' ability to clear abnormal proteins. He also studies the cells' regulation of RNA molecules created as part of expressing protein-encoding genes. Looking further upstream in the protein-producing process could yield further clues to why disease develops and what can be done about it, he says.