Jan 14 2015
One of nanotechnology’s greatest promises is interacting with the biological world the way our own cells do, but current biosensors must be tailor-made to detect the presence of one type of protein, the identity of which must be known in advance.
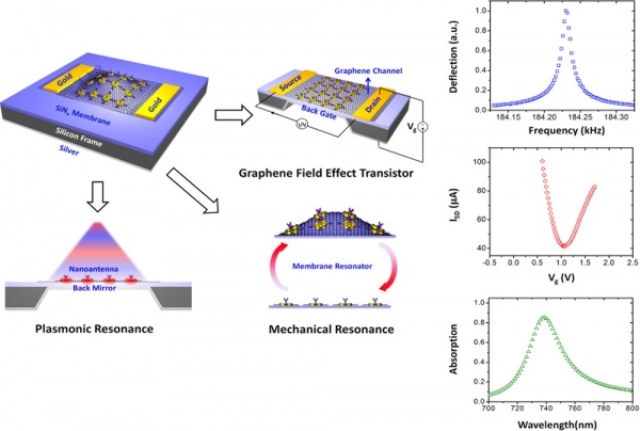 The researchers' biosensor has mechanical, electrical and optical modes.
The researchers' biosensor has mechanical, electrical and optical modes.
University of Pennsylvania engineers have now devised a new kind of graphene-based biosensor that works in three ways at once. Because proteins trigger three different types of signals, the sensor can triangulate this information to produce more sensitive and accurate results. By taking advantage of the unique integration of multiple physical sensing modes on the same chip, this sensor device can extend the protein-concentration sensing range by a thousand-fold.
This extended range could be particularly useful in early diagnosis of certain cancers, where the blood biomarker concentration varies by orders of magnitude from patient to patient. The ability to make multiple detections of the same biomarker on the same chip also has the potential to reduce false positives and negatives in medical diagnostic tests.
Eventually, such a technique could be used in an all-purpose biosensor, which could identify a wide range of proteins through their mass, as well as their optical and electrical properties.
A biosensor that did not have to be fine-tuned to detect only specific proteins would have a host of biomedical applications in diagnostic devices.
The study, published in the journal NanoLetters, was conducted by Ertugrul Cubukcu, assistant professor in the departments of Materials Science and Engineering and Electrical and Systems Engineering in Penn’s School of Engineering and Applied Science, and members of his lab, Alexander Y. Zhu, Fei Yi, Jason C. Reed and Hai Zhu.
“In a typical single mode biosensor you have two proteins that interact strongly. You attach protein A to your sensor and, when protein B binds to it, the sensor transduces that binding into some sort of electrical signal,” Cubukcu said,” But it's kind of a dumb sensor in that it can only tell you if that kind of binding has occurred.
“But let's say you have proteins A, B, C and D, all with different physical properties, like charge and mass. If you had a sensor that was sensitive to several of those properties, you could tell the difference between those binding events without starting with corresponding proteins for all of them.”
The more sensing modes operating at once, the better a sensor is at distinguishing between similar proteins. Proteins A and B might have the same mass but different charges, while proteins B and C have the same charges but different optical properties.
A multimodal sensor, pulling in data from multiple categories, could narrow the identity of a protein by comparing those values to a large database. Such an ability could potentially enable it to be applied to samples where the protein’s contents are unknown, a major upgrade on current technology which generally involves custom-building sensors to detect the presence of pre-defined sets of proteins.
The team’s sensors consist of a base of silicon nitride, coated with a layer of graphene, a single-atom-thick lattice of carbon atoms. Being carbon based means that graphene is an attractive bonding surface for proteins, which means that the device doesn’t need to be “functionalized” with proteins that are apt to interact with the ones the sensor aims to detect.
Graphene’s extreme thinness and unique electrical properties also allow for the mechanical, electrical and optical modes to operate simultaneously without interfering with one another.
“In the mechanical mode, the graphene is like the skin of a drum,” said Alexander Zhu, the first author of the study, who was then an undergraduate working in Cubukcu’s lab. “As proteins bind, the total mass changes and the resonance of the drum changes as a function of the total mass.
“In the electrical mode, we can look at how electrons travel across the graphene. The conductance is a function of the total available carriers inside, so, if you have something binding to the graphene, that changes the number of carriers and therefore the conductance properties.
“Finally, in the optical mode, we have a source of visible light and shine it on the sensor and measure the reflection. When nothing is bound, it’s seeing just air, but, as soon as proteins bind, we can measure the change in the refractive index.”
In their study, the researchers tested their sensor with known samples of proteins in order to demonstrate that all three modes can work simultaneously.
“We’ve shown that one sample provides all three shifts,” Yi said, “in the mass, electrical and optical readouts.”
Further work from Cubukcu’s group will investigate the feasibility of using this multimodal sensor to identify proteins from unknown samples.
The research was supported by the National Science Foundation under grants IIP-1312202 and ECCS-1408139.