Apr 27 2015
Functional analysis of a cell, which is the fundamental unit of life, is important for gaining new insights into medical and pharmaceutical fields. For efficiently studying cell functions, it is essential to reconstruct cellular microenvironments by parallel manipulation of single cells. Various cell manipulation techniques including fluidic, optical, and electrical techniques have been developed.
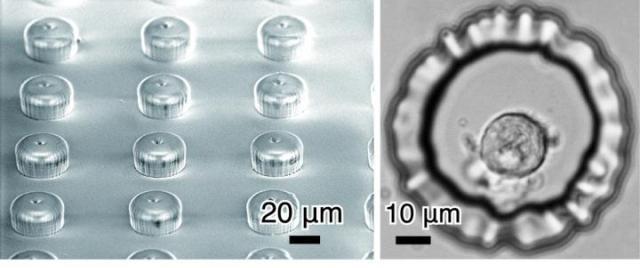 This shows a developed microprobe array and a single cell placed in a microwell.
This shows a developed microprobe array and a single cell placed in a microwell.
However, all these techniques lack flexibility with respect to changes in the cellular types, number, and places. In addition, the manipulations, which have been conducted in enclosed environments such as micro-channels, have limited access to the cells. It would be ideal if the working of a manipulation tool is analogous to grasping and transporting cells by one's fingers.
Now, researchers at Toyohashi Tech, at the Department of Mechanical Engineering, have developed a novel cell-manipulation tool that can trap and release single cells in a parallel arrangement in open-top microwells. The researchers fabricated an array of hollow microprobes to improve throughput and achieve flexibility in single-cell manipulation. The microprobes work like micro fingers picking up human cells. Single cells were trapped by suction and released by a flow generated through microchannels along the center of the probes.
"Parallel and versatile cell manipulation tools are essential for biomedical innovation", said Assistant Professor Moeto Nagai. "Microfabrication technologies offer massively parallel microstructures close to a human cell in size," he said.
"We fabricated an array of hollow microprobes with designed diameters, heights, and numbers from a silicon substrate using microfabrication techniques. Single cells were trapped on the tips of the probes using a suction flow. The cells were then released and placed in an array of microwells," Nagai said.
The research team developed a design principle for probes for minimally invasive single-cell manipulation. They conducted a cell aspiration experiment with a glass pipette and modeled a cell, thereby gaining information for designing hollow stepped probes. Based on the findings, the researchers designed and fabricated optimal probes. After a process of trial and error, the cells were placed and cultured in microwells with the probes, and the cells continued to maintain cell activity.
The proposed manipulation system makes it possible to place cells in a microwell array and observe the adherence, spreading, culture, and death of the cells. This system has the potential to be used as a tool for three-dimensional hetero cellular assays. In the future, this system will be further developed to reconstruct microenvironments of stem cells outside a living body, which would aid studies on stem cells for regenerative medicine.