May 20 2015
A research group at MANA, NIMS, succeeded in developing porous particles (mesoporous particles) consisting solely of phospholipids, a biological component, that are suitable for use as a drug delivery system.
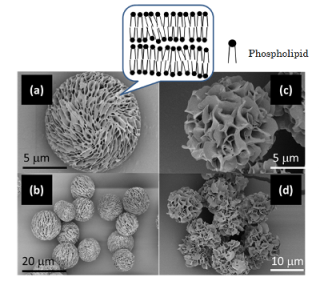 External views of porous phospholipid particles (electron micrographs). (a, b) Particles created in organic (non-aqueous) solvent, (c, d) particles created in organic solvent in the presence of small amount of water. Only hydrogenated soybean lecithin was used to create them. The shape of the particles varies greatly depending on the presence/absence of water in the solvent.
External views of porous phospholipid particles (electron micrographs). (a, b) Particles created in organic (non-aqueous) solvent, (c, d) particles created in organic solvent in the presence of small amount of water. Only hydrogenated soybean lecithin was used to create them. The shape of the particles varies greatly depending on the presence/absence of water in the solvent.
Abstract
- A research group led by MANA Scientist Kohsaku Kawakami, postdoctoral researcher Shaoling Zhang and MANA Principal Investigator Katsuhiko Ariga, at the International Center for Materials Nanoarchitectonics (MANA), NIMS (Sukekatsu Ushioda, President), succeeded in developing porous particles (mesoporous particles) consisting solely of phospholipids, a biological component, that are suitable for use as a drug delivery system. This study had been published in the Journal of Physical Chemistry C on 16 March, 2015. (Shaoling Zhang, Kohsaku Kawakami, Lok Kumar Shrestha, Gladstone Christopher Jayakumar, Jonathan P. Hill, and Katsuhiko Ariga, article title: Totally phospholipidic mesoporous particles) J. Phys. Chem. C, 2015, 119 (13), pp 7255–7263, DOI: 10.1021/acs.jpcc.5b00159.
- Mesoporous materials are a type of material capable of serving as a drug delivery system. In conventional studies, hard materials such as silica and carbon materials have been used for such purposes, posing safety concerns to patients. The mesoporous material developed in this study consists exclusively of biologically-derived materials and is therefore expected to be very safe for humans.
- Acquisition of official approval is one of the hurdles in the development of materials for use as a drug delivery system. To develop a certified pharmaceutical product, it is necessary to demonstrate the safety of the additive to be used before investigating the safety of the product itself. For this reason, pharmaceutical companies tend to avoid using new additives, which had been slowing the development of new drug carriers. However, the phospholipids examined in this study have already been used as emulsions and liposomes, and thus are not regarded as new additives. This fact is a great advantage of this material in view of commercialization.
- This material comprises highly uniform mesoporous particles with diameters ranging between 5 and 20 μm, depending on their composition. It is a very lightweight material with a bulk density of about 0.02 g/cm3, from which an aerodynamic diameter of 1 to 3 μm is calculated. These are ideal features for this material to be used as a powder inhalation carrier.
- Since this material consists of lipid bilayer membranes that are similar to biological membranes, it possesses the characteristics of both mesoporous particles and liposomes. For example, it can be used with both hydrophobic and hydrophilic drugs. Hydrophobic drugs can be embedded in a lipid bilayer membrane, and hydrophilic drugs can be inserted into hydrophilic regions between lipid bilayer membranes. Furthermore, as it is also feasible for the material to hold drugs in its mesopores, the material is capable of carrying drugs with various physical properties. Since phospholipids can be easily modified, it is conceivable that various kinds of surface modifications can be applied to the material.
- This material is suited for industrial production as it can be easily prepared through freeze-drying. And it is expected to be useful as a drug carrier assuming any administration route and as a cosmetic ingredient. The unique shape of the particles also may add value to the commercial product.
- This study had been published in the Journal of Physical Chemistry C issued by the American Chemical Society. It was also presented at the MANA Symposium at the Tsukuba International Congress Center (presentation: 3:20 p.m. on March 12, titled “Bio-inspired nanoarchitectonics for early and patient-oriented medical treatment” by K. Kawakami).