Aug 11 2015
Researchers at the Nanoparticles by Design Unit at the Okinawa Institute of Science and Technology Graduate University (OIST) have developed a novel method by encapsulating noble metal nanoparticles within a porous metal oxide shell to prevent them from compacting.
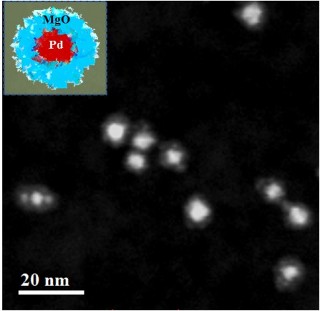 Electron Microscopy image depicting the Palladium- Magnesium Oxide core-shell combination. The white dots are Palladium nanoparticles. The slight haze around each nanoparticle is the porous magnesium oxide shell. The Palladium nanoparticles are not sintered together and maintain spaces between each another because of these shells. This maximizes their ability to react with chemicals.
Electron Microscopy image depicting the Palladium- Magnesium Oxide core-shell combination. The white dots are Palladium nanoparticles. The slight haze around each nanoparticle is the porous magnesium oxide shell. The Palladium nanoparticles are not sintered together and maintain spaces between each another because of these shells. This maximizes their ability to react with chemicals.
They conducted the research along with the SLAC National Laboratory in the USA and the Austrian Centre for Electron Microscopy and Nanoanalysis.
The latest breakthrough holds promise in the field of nano-catalysis for the development of more efficient fuel cells. The study has been published in Nanoscale.
Fuel cells contain electrical energy, which if directly harnessed from live and self-sustaining chemical reactions could provide low-cost alternatives to fossil fuels and help address global energy needs.
In order to promote rapid energy conversion in fuel cells, the researchers scattered nanoparticles that were made from noble metals such as silver, platinum and gold along an electrode’s surface.
However, unlike other metals, noble metals are not as chemically responsive at the macroscale, but their atoms tend to be more responsive at the nanoscale. Nanoparticles developed from such metals serve as a catalyst and improve the speed of the required chemical reaction, which releases electrons from the fuel.
Upon sputtering the nanoparticles on the electrode, they crush together similar to putty and form huge clusters. This compacting tendency is known as sintering, which decreases the entire surface area available to fuel molecules to react with the catalytic nanoparticles, and prevents the molecules from reaching their full potential in the fuel cells.
The researchers at OIST developed a new system by encapsulating palladium nanoparticles in a magnesium oxide shell and then dispersed this combination of core and shell on an electrode. They subsequently determined the abilities of the immersed electrode in enhancing the speed of the electrochemical reaction occurring in methanol fuel cells. The team showed that when compared to bare palladium nanoparticles, encapsulated palladium nanoparticles deliver excellent performance.
While examining palladium and magnesium nanoparticles separately, the research team had discovered that magnesium oxide nanoparticles can create permeable shells around nanoparticles made from noble metals.
The permeability of this extra protective covering ensures that fuel molecules are not prevented from reaching the encapsulated palladium. Images obtained through electron microscopy validated that the shell made of magnesium oxide serves as a spacer between the palladium cores when they attempt to adhere to each other, thus allowing each core to reach its full reactive potential.
Using OIST’s sophisticated nanoparticle deposition system, the researchers were easily able to modify the experimental parameters, vary the number of palldium nanoparticles in the core, and also vary the width of the encapsulating shell. Adjusting the structures and sizes of nanoparticles changes their chemical and physical properties for different types of applications.
“More core-shell combinations can be tried using our technique, with metals cheaper than Palladium for instance, like Nickel or Iron. Our results show enough promise to continue in this new direction,” stated Vidyadhar Singh, the paper’s first author and postdoctoral fellow under the supervision of Prof. Mukhles Sowwan, the director of OIST’s Nanoparticles by Design Unit; Sowwan was also the paper’s corresponding author.