Oct 15 2015
Brain tumor tissue can be hard to distinguish from normal brain during surgery. Neurosurgeons use their best judgment in the operating room but often must guess exactly where the edges of the tumor are while removing it.
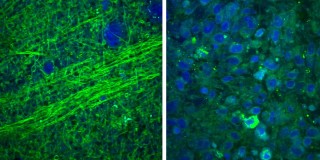 Images collected using an SRS microscope show that normal brain contains sparse cells with bundles of nerve fibers, called axons (left), but brain tumor tissue is full of cells in a disordered pattern (right). While you can see the difference on a microscopic scale, during surgery they’d be difficult to differentiate, making it hard for a surgeon to know where the tumor stops. Credit: University of Michigan Health System
Images collected using an SRS microscope show that normal brain contains sparse cells with bundles of nerve fibers, called axons (left), but brain tumor tissue is full of cells in a disordered pattern (right). While you can see the difference on a microscopic scale, during surgery they’d be difficult to differentiate, making it hard for a surgeon to know where the tumor stops. Credit: University of Michigan Health System
Even the state-of-the-art imaging equipment in today’s OR still doesn’t make the process much easier. But a new laser-based microscopic technology may help surgeons see the difference between tumor tissue and normal brain in real-time.
Called a stimulated Raman scattering (SRS) microscope, it’s now being tested at the University of Michigan Health System. Researchers have used the SRS microscope to image more than 60 patient samples since June – the first clinical test of the technology.
“It allows the surgical decision-making process to become data driven instead of relying on the surgeon’s best guess,” said Daniel Orringer, MD, the U-M neurosurgeon piloting the technology in collaboration with the Pathology Department at the University of Michigan Medical School. The team’s latest research was published today in Science Translational Medicine.
Working with experts from several institutions including Harvard University, where SRS microscopy was developed, to the U-M team uses SRS microscopes to image brain tissue from neurosurgical patients.
“We’re able to visualize tumor that otherwise would be invisible to the surgeon in the operating room,” Orringer said.
If the current test goes well, the technology could be submitted to the FDA for approval within two years.
The team developing the SRS microscope hopes to create a product about the size of a microwave and more affordable than the imaging systems currently used, such as intraoperative MRI. Orringer believes removing the cost and space-capacity barriers will make this technology appealing to many surgeons.
“This technology has the potential to resolve a long-standing issue in cancer surgery, which is the need for faster and more effective methods to assess whether a tumor has been fully removed,” said Richard Conroy, Ph.D., director of the Division of Applied Science & Technology at the National Institute of Biomedical Imaging and Bioengineering at the National Institutes of Health, which provided funding for the development of the technology.
As U-M’s Orringer and his colleagues continue testing the SRS microscope at U-M, they are building a second-generation system that can easily be operated during surgery.
The next version of the device would sit close to the operating table. Surgeons could readily insert a fresh tissue sample into the device, which would generate microscopic images on the spot. The entire medical team would be able to use and understand the device easily and the images produced would help determine immediately whether more surgery is required.
To get microscopic images today similar to what SRS produces, surgeons have to wait a half hour or more for tissue to be frozen, sectioned, stained and interpreted by expert pathologists trained to spot the difference between cancer cells and normal brain cells.
“The ability to determine tumor margins without having to send samples to a pathologist could increase patient safety and improve outcomes by shortening the length of surgeries and reducing the number of cases where cancer cells are left behind,” Conroy said.
“By optimizing surgical results, we’re ensuring that the patients will have the best possible outcomes after brain tumor surgery,” Orringer said.
Watch Orringer explain the technology.
Additional Authors:
- U-M’s Spencer Lewis, Sandra Camelo-Piragua, Sriram Venneti, Amanda Fisher-Hubbard, Mia Garrard, Anthony C. Wang, Jason A. Heth, Cormac O. Maher, Timothy D. Johnson, Oren Sagher
- Harvard’s Minbiao Ji, Shakti H. Ramkissoon, Dan Fu, and Xiaoliang Sunney Xie
- New York University’s Matija Snuderl
- Barrow Neurological Institute’s Nader Sanai
- Invenio Imaging’s Christian W. Freudiger
Funding:
- National Institute of Biomedical Imaging and Bioengineering (R01EB017254)
- National Cancer Institute (R01CA175391)
- National Institute of Neurologic Disorders and Stroke (K08NS087118; F32NS074744)
- National Institute of Health’s Director’s Transformative Research Award Program T-R01 (R01EB010244-01)
- 2013-2014 American Association of Neurological Surgeons NREF Young Clinician Investigator Award
- Michigan Institute for Clinical and Health Research (2UL1TR000433)
Disclosure: Orringer and Xie are advisors and shareholders of Invenio Imaging, Inc., a company developing SRS microscopy systems. Freudiger is an employee of Invenio Imaging, Inc.
The University of Michigan, Harvard University and Invenio Imaging have intellectual property used in this research.
Reference: “Detection of human brain tumor infiltration with quantitative stimulated Raman scattering microscopy,” Science Translational Medicine, 14 Oct., 2015.