May 24 2016
Scientists at Cornell University in Ithaca, New York have developed a novel drug delivery mechanism that uses two separate vehicles, permitting the delivery of physically and chemically different agents. This mechanism was used to deliver a unique anti-cancer combination therapy containing piperlongumine (PL) and TRAIL for treatment of HCT116 colon cancer cells and PC3 prostate cancer.
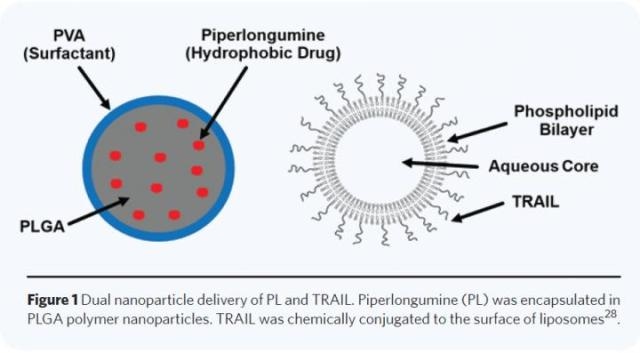 Piperlongumine (PL) was encapsulated in PLGA polymer nanoparticles. TRAIL was chemically conjugated to the surface of liposomes. (Credit- Technology)
Piperlongumine (PL) was encapsulated in PLGA polymer nanoparticles. TRAIL was chemically conjugated to the surface of liposomes. (Credit- Technology)
PL, which is a tiny-molecule hydrophobic drug, was embedded in poly (lactic-co-glycolic acid) (PLGA) nanoparticles, while TRAIL was conjugated chemically to the surface of nanoscale liposomes. PL was initially injected to expose tumor cells to the consequences of TRAIL. HCT116 and PC3 cells had lower survival rates in vitro after getting the combination nanoparticle therapy in comparison to every agent separately.
In vivo testing was concerned with a subcutaneous xenograft mouse model using HCT116 cells and NOD-SCID gamma mice. Two treatment cycles were administered for a period of 48 hours. HCT116 cancer cells that underwent the dual or two-stage nanoparticle therapy had higher apoptotic rates compared to separate stages of the nanoparticle therapy alone. The report has been published in the current issue of the TECHNOLOGY journal.
We have found that liposomal TRAIL shows great promise for the destruction of tumor cells in the bloodstream, lymphatic system, and also in solid tumors.
Daljit S. and Elaine Sarkaria Professor Michael R. King, Ph.D., Cornell University
In the paper, the scientists explained a drug delivery mechanism based on nanoparticles, specifically created for a combination cancer therapy. Nanoparticle-based delivery platforms have been previouslystudied for various cancer therapies. Numerous studies have concentrated on complicated particle structures and designs focused at enhancing delivery.
The system developed by the Cornell team uses another approach, making use of a double nanoparticle delivery mechanism. Therapeutic compounds were given through separate particles in contrast to delivering the agents as a lone particle. The drug was encapsulated within polymer-based nanoparticles due to the hydrophobic nature of the TRAIL sensitizer.
In the project, the scientists saw a greater number of apoptotic cells in mouse trials, reliable with their observed lab findings that showed an improved therapeutic efficiency while using the dual nanoparticle therapy in comparison to individual stages of nanoparticle therapy alone. The dual nanoparticle delivery system gave a certain level of flexibility while administering both stages of the therapy, permitting an opportunity for cancer cell sensitization before administering the therapy’s second component.
The Cornell group is currently working to form a unique therapeutic method to target tumor cells within the bloodstream for preventing prostate cancer metastasis in which circulating leukocytes are used as a vehicle for TRAIL, the apoptosis ligand. After being introduced in the bloodstream, E-selectin/TRAIL liposomes bind to the peripheral blood leukocytes’ surface, to make these "unnatural killer cells" cytotoxic to the circulating tumor cells without impacting either the endothelial cell or blood cell viability. The researchers earlier proved that viable tumor cells could be quickly cleared from the bloodstream by using concentrations of liposome-bound TRAIL that are of magnitude twice lesser than the dosages employed in previous human clinical trials of soluble TRAIL protein. Recently, it has been discovered that E-selectin/TRAIL liposomes could fully obstruct metastasis and also reduce the size of primary prostate cancer tumors in mice.